Meta-analysis shows no consistent evidence for senescence in ejaculate traits across animals
- PMID: 38228708
- PMCID: PMC10791739
- DOI: 10.1038/s41467-024-44768-4
Meta-analysis shows no consistent evidence for senescence in ejaculate traits across animals
Abstract
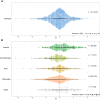
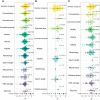
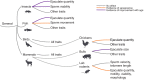
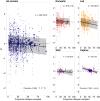
Male reproductive traits such as ejaculate size and quality, are expected to decline with advancing age due to senescence. It is however unclear whether this expectation is upheld across taxa. We perform a meta-analysis on 379 studies, to quantify the effects of advancing male age on ejaculate traits across 157 species of non-human animals. Contrary to predictions, we find no consistent pattern of age-dependent changes in ejaculate traits. This result partly reflects methodological limitations, such as studies sampling a low proportion of adult lifespan, or the inability of meta-analytical approaches to document non-linear ageing trajectories of ejaculate traits; which could potentially lead to an underestimation of senescence. Yet, we find taxon-specific differences in patterns of ejaculate senescence. For instance, older males produce less motile and slower sperm in ray-finned fishes, but larger ejaculates in insects, compared to younger males. Notably, lab rodents show senescence in most ejaculate traits measured. Our study challenges the notion of universal reproductive senescence, highlighting the need for controlled methodologies and a more nuanced understanding of reproductive senescence, cognisant of taxon-specific biology, experimental design, selection pressures, and life-history.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Ejaculate components delay reproductive senescence while elevating female reproductive rate in an insect.Proc Natl Acad Sci U S A. 2009 Dec 22;106(51):21743-7. doi: 10.1073/pnas.0905347106. Epub 2009 Dec 8. Proc Natl Acad Sci U S A. 2009. PMID: 19996174 Free PMC article.
-
Effects of nutrient limitation on sperm and seminal fluid: a systematic review and meta-analysis.Biol Rev Camb Philos Soc. 2019 Oct;94(5):1722-1739. doi: 10.1111/brv.12524. Epub 2019 Jun 19. Biol Rev Camb Philos Soc. 2019. PMID: 31215758
-
Male alternative reproductive tactics and sperm competition: a meta-analysis.Biol Rev Camb Philos Soc. 2022 Aug;97(4):1365-1388. doi: 10.1111/brv.12846. Epub 2022 Feb 28. Biol Rev Camb Philos Soc. 2022. PMID: 35229450 Free PMC article.
-
Condition-dependent ejaculate size and composition in a ladybird beetle.Proc Biol Sci. 2010 Dec 7;277(1700):3639-47. doi: 10.1098/rspb.2010.0810. Epub 2010 Jun 23. Proc Biol Sci. 2010. PMID: 20573622 Free PMC article.
-
Intra-specific correlations between ejaculate traits and competitive fertilization success: a meta-analysis across species and fertilization modes.Evolution. 2024 Feb 29;78(3):497-510. doi: 10.1093/evolut/qpad229. Evolution. 2024. PMID: 38146674
Cited by
-
Influences of aging and mating history in males on paternity success in the red flour beetle Tribolium castaneum.PLoS One. 2024 Dec 23;19(12):e0316008. doi: 10.1371/journal.pone.0316008. eCollection 2024. PLoS One. 2024. PMID: 39715202 Free PMC article.
-
Reproductive output of old males is limited by seminal fluid, not sperm number.Evol Lett. 2025 Jan 6;9(2):282-291. doi: 10.1093/evlett/qrae071. eCollection 2025 Apr. Evol Lett. 2025. PMID: 40191416 Free PMC article.
-
What does not kill you makes you stronger? Effects of paternal age at conception on fathers and sons.Evolution. 2024 Sep 3;78(9):1619-1632. doi: 10.1093/evolut/qpae097. Evolution. 2024. PMID: 38912848 Free PMC article.
-
Genetic variation in age-dependent attractiveness in a fish with a mixed mating system.Biol Lett. 2025 Jan;21(1):20240448. doi: 10.1098/rsbl.2024.0448. Epub 2025 Jan 22. Biol Lett. 2025. PMID: 39838734
-
Age-dependent decline in sperm quality and function in a naturally short-lived vertebrate.BMC Ecol Evol. 2025 Jan 6;25(1):2. doi: 10.1186/s12862-024-02343-x. BMC Ecol Evol. 2025. PMID: 39762763 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

