Retention rate of subcutaneous TNF inhibitors in axial spondyloarthritis in a multicentre study from the RIC-FRANCE network
- PMID: 38228719
- PMCID: PMC10791989
- DOI: 10.1038/s41598-024-52016-4
Retention rate of subcutaneous TNF inhibitors in axial spondyloarthritis in a multicentre study from the RIC-FRANCE network
Abstract
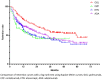
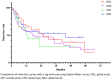
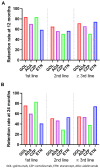
The objectives of our study were to assess retention rate, safety, and predictive factors for retention of subcutaneous (SC) TNF inhibitors (TNFi) (adalimumab (ADA), etanercept (ETN), golimumab (GOL), and certolizumab pegol (CZP)) in axial spondyloarthritis (axSpA) depending on the line of treatment in real-life conditions. A multicentre retrospective observational study was conducted including 552 patients fulfilling the ASAS criteria for axSpA followed in the RIC-France register who began SC-TNFi between 01/01/13 and 08/31/2018 for a total of 824 prescriptions. Taking all lines of treatment into account, GOL had a significantly higher retention rate compared with ADA, ETN, and CZP with a mean retention length of 59 months. As first-line bDMARDs, GOL had a significantly higher retention rate compared with ADA and ETN. ETN had the best retention rate when prescribed as at least 3rd bDMARD. Taking all lines of treatment into account, female sex, peripheral disease, BASDAI at initiation, and line of treatment were predictive factors for treatment cessation. Primary inefficiency was the most frequent reason for treatment cessation. In conclusion, GOL showed the highest retention rate in axSpA. Male sex, absence of peripheral disease, and early line of prescription were associated with better SC-TNFi retention in axSpA.
© 2024. The Author(s).
Conflict of interest statement
GL: NOVARTIS, GSK, AMGEN VG: MSD, UCB, Pfizer, Sanofi , novartis, Abbvie, Medac JHS: AbbVie, BMS, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB, Viatris RMF: Abbvie, Pfizer, MSD, Celltrion EG: BMS, Sanofi-Aventis, Roche, Abbvie, Novartis, Pfizer, Galapagos, MSD, Janssen FM, GB, GTD, PQ, EH, EV, LM, MHG had no competing interest.
Figures


Similar articles
-
Retention rates of adalimumab, etanercept, and infliximab as first- or second-line biotherapies for spondyloarthritis patients in daily practice in Auvergne (France).Int J Rheum Dis. 2018 Nov;21(11):1986-1992. doi: 10.1111/1756-185X.13375. Epub 2018 Aug 30. Int J Rheum Dis. 2018. PMID: 30168265 Free PMC article.
-
High BMI is associated with lower TNF-α inhibitor serum trough levels and higher disease activity in patients with axial spondyloarthritis.Arthritis Res Ther. 2023 Oct 17;25(1):202. doi: 10.1186/s13075-023-03187-4. Arthritis Res Ther. 2023. PMID: 37848964 Free PMC article.
-
Inflammatory hallmarks of lesser prominence in psoriatic arthritis patients starting biologics: a Nordic population-based cohort study.Rheumatology (Oxford). 2021 Jan 5;60(1):140-146. doi: 10.1093/rheumatology/keaa237. Rheumatology (Oxford). 2021. PMID: 32591790
-
Certolizumab pegol for treating axial spondyloarthritis.Expert Opin Biol Ther. 2016 Aug;16(8):1059-64. doi: 10.1080/14712598.2016.1205581. Expert Opin Biol Ther. 2016. PMID: 27366922 Review.
-
Longterm Drug Survival of Tumor Necrosis Factor Inhibitors in Patients with Rheumatoid Arthritis.J Rheumatol. 2020 Apr;47(4):493-501. doi: 10.3899/jrheum.181398. Epub 2019 Jun 1. J Rheumatol. 2020. PMID: 31154413
Cited by
-
Analysis of the shorter drug survival times for Janus kinase inhibitors and interleukin-17 inhibitors compared with tumor necrosis factor inhibitors in a real-world cohort of axial spondyloarthritis patients - a retrospective analysis from the RHADAR network.Rheumatol Int. 2024 Oct;44(10):2057-2066. doi: 10.1007/s00296-024-05671-9. Epub 2024 Aug 13. Rheumatol Int. 2024. PMID: 39136784 Free PMC article.
-
Real-World Adherence and Drug Survival of Biologics among Patients with Ankylosing Spondylitis.J Clin Med. 2024 Jul 31;13(15):4480. doi: 10.3390/jcm13154480. J Clin Med. 2024. PMID: 39124747 Free PMC article.
References
-
- Sepriano A, et al. Efficacy and safety of biological and targeted-synthetic DMARDs: A systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000396. doi: 10.1136/rmdopen-2016-000396. - DOI - PMC - PubMed
-
- Svedbom A, Storck C, Kachroo S, Govoni M, Khalifa A. Persistence with golimumab in immune-mediated rheumatic diseases: a systematic review of real-world evidence in rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis. Patient Prefer. Adherence. 2017;11:719–729. doi: 10.2147/PPA.S128665. - DOI - PMC - PubMed
-
- Kristensen LE, et al. Presence of peripheral arthritis and male sex predicting continuation of anti-tumor necrosis factor therapy in ankylosing spondylitis: An observational prospective cohort study from the South Swedish arthritis treatment group register. Arthritis Care Res. 2010;62:1362–1369. doi: 10.1002/acr.20258. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

