Dopamine neuron degeneration in the Ventral Tegmental Area causes hippocampal hyperexcitability in experimental Alzheimer's Disease
- PMID: 38228889
- PMCID: PMC11189820
- DOI: 10.1038/s41380-024-02408-9
Dopamine neuron degeneration in the Ventral Tegmental Area causes hippocampal hyperexcitability in experimental Alzheimer's Disease
Abstract
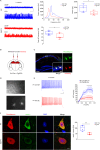
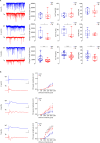
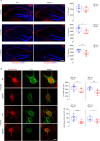
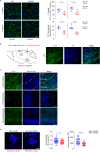
Early and progressive dysfunctions of the dopaminergic system from the Ventral Tegmental Area (VTA) have been described in Alzheimer's Disease (AD). During the long pre-symptomatic phase, alterations in the function of Parvalbumin interneurons (PV-INs) are also observed, resulting in cortical hyperexcitability represented by subclinical epilepsy and aberrant gamma-oscillations. However, it is unknown whether the dopaminergic deficits contribute to brain hyperexcitability in AD. Here, using the Tg2576 mouse model of AD, we prove that reduced hippocampal dopaminergic innervation, due to VTA dopamine neuron degeneration, impairs PV-IN firing and gamma-waves, weakens the inhibition of pyramidal neurons and induces hippocampal hyperexcitability via lower D2-receptor-mediated activation of the CREB-pathway. These alterations coincide with reduced PV-IN numbers and Perineuronal Net density. Importantly, L-DOPA and the selective D2-receptor agonist quinpirole rescue p-CREB levels and improve the PV-IN-mediated inhibition, thus reducing hyperexcitability. Moreover, similarly to quinpirole, sumanirole - another D2-receptor agonist and a known anticonvulsant - not only increases p-CREB levels in PV-INs but also restores gamma-oscillations in Tg2576 mice. Conversely, blocking the dopaminergic transmission with sulpiride (a D2-like receptor antagonist) in WT mice reduces p-CREB levels in PV-INs, mimicking what occurs in Tg2576. Overall, these findings support the hypothesis that the VTA dopaminergic system integrity plays a key role in hippocampal PV-IN function and survival, disclosing a relevant contribution of the reduced dopaminergic tone to aberrant gamma-waves, hippocampal hyperexcitability and epileptiform activity in early AD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
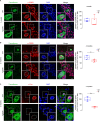
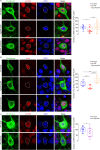
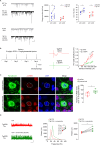
Similar articles
-
Early derailment of firing properties in CA1 pyramidal cells of the ventral hippocampus in an Alzheimer's disease mouse model.Exp Neurol. 2022 Apr;350:113969. doi: 10.1016/j.expneurol.2021.113969. Epub 2021 Dec 31. Exp Neurol. 2022. PMID: 34973962
-
Dopamine loss alters the hippocampus-nucleus accumbens synaptic transmission in the Tg2576 mouse model of Alzheimer's disease.Neurobiol Dis. 2018 Aug;116:142-154. doi: 10.1016/j.nbd.2018.05.006. Epub 2018 May 17. Neurobiol Dis. 2018. PMID: 29778899
-
Repetitive prefrontal tDCS activates VTA dopaminergic neurons, resulting in attenuation of Alzheimer's Disease-like deficits in Tg2576 mice.Alzheimers Res Ther. 2025 Apr 29;17(1):94. doi: 10.1186/s13195-025-01736-4. Alzheimers Res Ther. 2025. PMID: 40301905 Free PMC article.
-
Parvalbumin Interneuron Dysfunction in Neurological Disorders: Focus on Epilepsy and Alzheimer's Disease.Int J Mol Sci. 2024 May 19;25(10):5549. doi: 10.3390/ijms25105549. Int J Mol Sci. 2024. PMID: 38791587 Free PMC article. Review.
-
The role of parvalbumin interneuron dysfunction across neurodegenerative dementias.Ageing Res Rev. 2024 Nov;101:102509. doi: 10.1016/j.arr.2024.102509. Epub 2024 Sep 19. Ageing Res Rev. 2024. PMID: 39306248 Review.
Cited by
-
Pharmacological reduction of reverse-translated hippocampal hyperactivity in mouse: relevance for psychosis.Neuropsychopharmacology. 2025 Jul;50(8):1265-1274. doi: 10.1038/s41386-025-02077-4. Epub 2025 Feb 27. Neuropsychopharmacology. 2025. PMID: 40016366
-
How dopamine tunes parvalbumin interneurons in the hippocampus: new experimental observations in Alzheimer's disease.Neural Regen Res. 2025 May 1;20(5):1405-1406. doi: 10.4103/NRR.NRR-D-24-00322. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 39075905 Free PMC article. No abstract available.
-
Gut microbiota metabolites: potential therapeutic targets for Alzheimer's disease?Front Pharmacol. 2024 Sep 17;15:1459655. doi: 10.3389/fphar.2024.1459655. eCollection 2024. Front Pharmacol. 2024. PMID: 39355779 Free PMC article. Review.
-
Dopamine Dilemma Overexcites the Hippocampus in Alzheimer's Disease.Epilepsy Curr. 2024 Oct 15;24(5):361-363. doi: 10.1177/15357597241280475. eCollection 2024 Sep-Oct. Epilepsy Curr. 2024. PMID: 39508014 Free PMC article. No abstract available.
-
Prophylactic treatment with the c-Abl inhibitor, neurotinib, diminishes neuronal damage and the convulsive state in pilocarpine-induced mice.Cell Rep. 2024 May 28;43(5):114144. doi: 10.1016/j.celrep.2024.114144. Epub 2024 Apr 23. Cell Rep. 2024. PMID: 38656874 Free PMC article.
References
-
- Gauthier S, Webster C, Servaes S, Morais JA, Rosa-Neto P. World Alzheimer Report 2022 – Life after diagnosis: Navigating treatment, care and support. London, England: Alzheimer’s Disease International; 2022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

