Attenuation of neurovirulence of chikungunya virus by a single amino acid mutation in viral E2 envelope protein
- PMID: 38229040
- PMCID: PMC10792792
- DOI: 10.1186/s12929-024-00995-x
Attenuation of neurovirulence of chikungunya virus by a single amino acid mutation in viral E2 envelope protein
Abstract
Background: Chikungunya virus (CHIKV) has reemerged as a major public health concern, causing chikungunya fever with increasing cases and neurological complications.
Methods: In the present study, we investigated a low-passage human isolate of the East/ Central/South African (ECSA) lineage of CHIKV strain LK(EH)CH6708, which exhibited a mix of small and large viral plaques. The small and large plaque variants were isolated and designated as CHIKV-SP and CHIKV-BP, respectively. CHIKV-SP and CHIKV-BP were characterized in vitro and in vivo to compare their virus production and virulence. Additionally, whole viral genome analysis and reverse genetics were employed to identify genomic virulence factors.
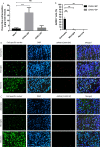
Results: CHIKV-SP demonstrated lower virus production in mammalian cells and attenuated virulence in a murine model. On the other hand, CHIKV-BP induced higher pro-inflammatory cytokine levels, compromised the integrity of the blood-brain barrier, and led to astrocyte infection in mouse brains. Furthermore, the CHIKV-SP variant had limited transmission potential in Aedes albopictus mosquitoes, likely due to restricted dissemination. Whole viral genome analysis revealed multiple genetic mutations in the CHIKV-SP variant, including a Glycine (G) to Arginine (R) mutation at position 55 in the viral E2 glycoprotein. Reverse genetics experiments confirmed that the E2-G55R mutation alone was sufficient to reduce virus production in vitro and virulence in mice.
Conclusions: These findings highlight the attenuating effects of the E2-G55R mutation on CHIKV pathogenicity and neurovirulence and emphasize the importance of monitoring this mutation in natural infections.
Keywords: Attenuation; Chikungunya virus; Neurovirulence; Pathogenesis; Plaque size.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

