MANF protein expression is upregulated in immune cells in the ischemic human brain and systemic recombinant MANF delivery in rat ischemic stroke model demonstrates anti-inflammatory effects
- PMID: 38229173
- PMCID: PMC10792833
- DOI: 10.1186/s40478-023-01701-y
MANF protein expression is upregulated in immune cells in the ischemic human brain and systemic recombinant MANF delivery in rat ischemic stroke model demonstrates anti-inflammatory effects
Abstract
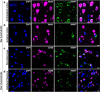
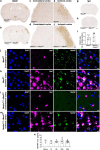
Mesencephalic astrocyte-derived neurotrophic factor (MANF) has cytoprotective effects on various injuries, including cerebral ischemia, and it can promote recovery even when delivered intracranially several days after ischemic stroke. In the uninjured rodent brain, MANF protein is expressed almost exclusively in neurons, but post-ischemic MANF expression has not been characterized. We aimed to investigate how endogenous cerebral MANF protein expression evolves in infarcted human brains and rodent ischemic stroke models. During infarct progression, the cerebral MANF expression pattern both in human and rat brains shifted drastically from neurons to expression in inflammatory cells. Intense MANF immunoreactivity took place in phagocytic microglia/macrophages in the ischemic territory, peaking at two weeks post-stroke in human and one-week post-stroke in rat ischemic cortex. Using double immunofluorescence and mice lacking MANF gene and protein from neuronal stem cells, neurons, astrocytes, and oligodendrocytes, we verified that MANF expression was induced in microglia/macrophage cells in the ischemic hemisphere. Embarking on the drastic expression transition towards inflammatory cells and the impact of blood-borne inflammation in stroke, we hypothesized that exogenously delivered MANF protein can modulate tissue recovery processes. In an attempt to enhance recovery, we designed a set of proof-of-concept studies using systemic delivery of recombinant MANF in a rat model of cortical ischemic stroke. Intranasal recombinant MANF treatment decreased infarct volume and reduced the severity of neurological deficits. Intravenous recombinant MANF treatment decreased the levels of pro-inflammatory cytokines and increased the levels of anti-inflammatory cytokine IL-10 in the infarcted cortex one-day post-stroke. In conclusion, MANF protein expression is induced in activated microglia/macrophage cells in infarcted human and rodent brains, and this could implicate MANF's involvement in the regulation of post-stroke inflammation in patients and experimental animals. Moreover, systemic delivery of recombinant MANF shows promising immunomodulatory effects and therapeutic potential in experimental ischemic stroke.
Keywords: Distal middle cerebral artery occlusion; Inflammation; Ischemia; Mesencephalic astrocyte-derived neurotrophic factor; Neuroprotection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare there are no conflicts of interest.
Figures





References
-
- Abo-Ramadan U, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, Soinne L, Strbian D, Tatlisumak T. Post-ischemic leakiness of the blood-brain barrier: a quantitative and systematic assessment by Patlak plots. Exp Neurol. 2009;219:328–333. doi: 10.1016/j.expneurol.2009.06.002. - DOI - PubMed
-
- Airavaara M, Chiocco MJ, Howard DB, Zuchowski KL, Peranen J, Liu C, Fang S, Hoffer BJ, Wang Y, Harvey BK. Widespread cortical expression of MANF by AAV serotype 7: localization and protection against ischemic brain injury. Exp Neurol. 2010;225:104–113. doi: 10.1016/j.expneurol.2010.05.020. - DOI - PMC - PubMed
-
- Anttila JE, Albert K, Wires ES, Matlik K, Loram LC, Watkins LR, Rice KC, Wang Y, Harvey BK, Airavaara M. Post-stroke intranasal (+)-naloxone delivery reduces microglial activation and improves behavioral recovery from ischemic injury. eNeuro. 2018 doi: 10.1523/ENEURO.0395-17.2018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

