Hypoxia and Singlet Oxygen Dual-Responsive Micelles for Photodynamic and Chemotherapy Therapy Featured with Enhanced Cellular Uptake and Triggered Cargo Delivery
- PMID: 38229704
- PMCID: PMC10790668
- DOI: 10.2147/IJN.S432407
Hypoxia and Singlet Oxygen Dual-Responsive Micelles for Photodynamic and Chemotherapy Therapy Featured with Enhanced Cellular Uptake and Triggered Cargo Delivery
Abstract
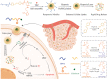
Introduction: Combination therapy provides better outcomes than a single therapy and becomes an efficient strategy for cancer treatment. In this study, we designed a hypoxia- and singlet oxygen-responsive polymeric micelles which contain azo and nitroimidazole groups for enhanced cellular uptake, repaid cargo release, and codelivery of photosensitizer Ce6 and hypoxia-activated prodrug tirapazamine TPZ (DHM-Ce6@TPZ), which could be used for combining Ce6-mediated photodynamic therapy (PDT) and PDT-activated chemotherapy to enhance the therapy effect of cancer.
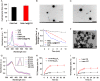
Methods: The hypoxia- and singlet oxygen-responsive polymeric micelles DHM-Ce6@TPZ were prepared by film hydration method. The morphology, physicochemical properties, stimuli responsiveness, in vitro singlet oxygen production, cellular uptake, and cell viability were evaluated. In addition, the in vivo therapeutic effects of the micelles were verified using a tumor xenograft mice model.
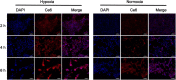
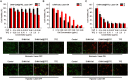
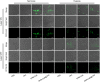
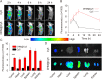
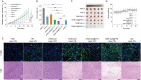
Results: The resulting dual-responsive micelles not only increased the concentration of intracellular photosensitizer and TPZ, but also facilitated photosensitizer and TPZ release for enhanced integration of photodynamic and chemotherapy therapy. As a photosensitizer, Ce6 induced PDT by generating toxic singlet reactive oxygen species (ROS), resulting in a hypoxic tumor environment to activate the prodrug TPZ to achieve efficient chemotherapy, thereby evoking a synergistic photodynamic and chemotherapy therapeutic effect. The cascade synergistic therapeutic effect of DHM-Ce6@TPZ was effectively evaluated both in vitro and in vivo to inhibit tumor growth in a breast cancer mice model.
Conclusion: The designed multifunctional micellar nano platform could be a convenient and powerful vehicle for the efficient co-delivery of photosensitizers and chemical drugs for enhanced synergistic photodynamic and chemotherapy therapeutic effect of cancer.
Keywords: combination therapy; hypoxia-responsive; photodynamic therapy; singlet oxygen-responsive.
© 2024 Guo et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Supramolecular micelles as multifunctional theranostic agents for synergistic photodynamic therapy and hypoxia-activated chemotherapy.Acta Biomater. 2021 Sep 1;131:483-492. doi: 10.1016/j.actbio.2021.07.014. Epub 2021 Jul 13. Acta Biomater. 2021. PMID: 34265471
-
Multi-stimuli responsive polymeric prodrug micelles for combined chemotherapy and photodynamic therapy.J Mater Chem B. 2020 Jun 24;8(24):5267-5279. doi: 10.1039/d0tb00539h. J Mater Chem B. 2020. PMID: 32441291
-
Multifunctional Micelles Dually Responsive to Hypoxia and Singlet Oxygen: Enhanced Photodynamic Therapy via Interactively Triggered Photosensitizer Delivery.ACS Appl Mater Interfaces. 2018 May 23;10(20):17117-17128. doi: 10.1021/acsami.8b06299. Epub 2018 May 11. ACS Appl Mater Interfaces. 2018. PMID: 29722261
-
Recent progress of porphyrin metal-organic frameworks for combined photodynamic therapy and hypoxia-activated chemotherapy.Chem Commun (Camb). 2024 Nov 19;60(93):13641-13652. doi: 10.1039/d4cc04512b. Chem Commun (Camb). 2024. PMID: 39497649 Review.
-
A comprehensive review on singlet oxygen generation in nanomaterials and conjugated polymers for photodynamic therapy in the treatment of cancer.Nanoscale. 2024 Feb 15;16(7):3243-3268. doi: 10.1039/d3nr05801h. Nanoscale. 2024. PMID: 38265094 Review.
Cited by
-
Evolution of nMOFs in photodynamic therapy: from porphyrins to chlorins and bacteriochlorins for better efficacy.Front Pharmacol. 2025 Mar 18;16:1533040. doi: 10.3389/fphar.2025.1533040. eCollection 2025. Front Pharmacol. 2025. PMID: 40170725 Free PMC article. Review.
-
Nanofibers in Glioma Therapy: Advances, Applications, and Overcoming Challenges.Int J Nanomedicine. 2025 Apr 14;20:4677-4703. doi: 10.2147/IJN.S510363. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40255668 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

