Immunosuppression with Generics in Liver and Kidney Transplantation: A Real-World Evidence Study
- PMID: 38229916
- PMCID: PMC10790661
- DOI: 10.2147/DDDT.S431121
Immunosuppression with Generics in Liver and Kidney Transplantation: A Real-World Evidence Study
Abstract
Purpose: This study evaluates the use, benefit-risk profile, and economic impact of generic immunosuppressants (tacrolimus-TAC, cyclosporine-CsA, and mycophenolate-MYC) in kidney and liver transplant recipients compared to brand-name drugs.
Patients and methods: A retrospective multicentre observational study, involving four Italian regions, was conducted based on the national transplant Information system and regional healthcare claims data. The analysis focused on incident patients who received kidney and liver transplants between 2013 and 2019 and evaluated the use of generic of CsA, TAC, and MYC during the 30-day period following discharge. For each type of transplant and immunosuppressive agent, the benefit-risk profile of generic vs branded drugs in a two-year window was estimated by multivariate Cox models (HR; 95% CI). Furthermore, the potential cost savings per person associated with one year of treatment using generics were calculated.
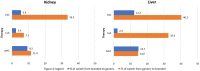
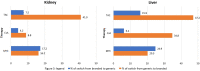
Results: The utilization of generic drugs showed a significant increase; over the study years, the proportion of users among kidney recipients ranged from 14.2% to 40.5% for TAC, from 36.9% to 56.7% for MYC, and from 18.2% to 94.7% for CsA. A great variability in generic uptake for region was found. A comparable risk-benefit profile between generic and branded formulations was shown for all immunosuppressors considered. Choosing generic immunosuppressants during maintenance could result in yearly savings of around 2000 euros per person for each therapy ingredient.
Conclusion: The study shows an increasing proportion of patients using generic immunosuppressive drugs over time suggesting a growing acceptance of generics within the transplant community and reveals comparable risk-benefit profiles between the generic and branded formulations of TAC, CsA, and MYC. A significant variability in the use of generics immunosuppressive agents was found both at the regional level and among transplant centers and future research should delve into regional prescribing variations.
Keywords: cyclosporine; maintenance therapy; mycophenolate; risk-benefit profile; sustainability; tacrolimus; transplant; uptake generics.
© 2024 Finocchietti et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





References
-
- AIFA. Determinazione AIFA; 2016. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/Determinazione_AIFA.... Accessed January 5, 2024.
-
- AIFA. Precisazioni AIFA su Tacrolimus; 2011. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/precisazioni_aifa_s.... Accessed January 5, 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

