Immunoglobulin A response to SARS-CoV-2 infection and immunity
- PMID: 38230244
- PMCID: PMC10789627
- DOI: 10.1016/j.heliyon.2024.e24031
Immunoglobulin A response to SARS-CoV-2 infection and immunity
Abstract
The novel coronavirus disease (COVID-19) and its infamous "Variants" of the etiological agent termed Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) has proven to be a global health concern. The three antibodies, IgA, IgM, and IgG, perform their dedicated role as main workhorses of the host adaptive immune system in virus neutralization. Immunoglobulin-A (IgA), also known as "Mucosal Immunoglobulin", has been under keen interest throughout the viral infection cycle. Its importance lies because IgA is predominant mucosal antibody and SARS family viruses primarily infect the mucosal surfaces of human respiratory tract. Therefore, IgA can be considered a diagnostic and prognostic marker and an active infection biomarker for SARS CoV-2 infection. Along with molecular analyses, serological tests, including IgA detection tests, are gaining ground in application as an early detectable marker and as a minimally invasive detection strategy. In the current review, it was emphasized the role of IgA response in diagnosis, host defense strategies, treatment, and prevention of SARS-CoV-2 infection. The data analysis was performed through almost 100 published peer-reviewed research reports and comprehended the importance of IgA in antiviral immunity against SARS-CoV-2 and other related respiratory viruses. Taken together, it is concluded that secretory IgA- Abs can serve as a promising detection tool for respiratory viral diagnosis and treatment parallel to IgG-based therapeutics and diagnostics. Vaccine candidates that target and trigger mucosal immune response may also be employed in future dimensions of research against other respiratory viruses.
Keywords: COVID-19; Immune response; Immunoglobulin A (IgA); Mucosal immunity; SARS-CoV-2; Serological test; Vaccine.
© 2024 The Author(s).
Conflict of interest statement
No competing interests to be declared.
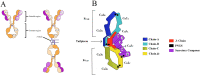
Figures


References
-
- Feng W., Newbigging A.M., Le C., Pang B., Peng H., Cao Y., et al. Molecular diagnosis of COVID-19: challenges and research needs. Anal. Chem. 2020;92(15):10196–10209. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

