Comparison between high-flow nasal cannula and conventional oxygen therapy in COVID-19 patients: a systematic review and meta-analysis
- PMID: 38230522
- PMCID: PMC10798115
- DOI: 10.1177/17534666231225323
Comparison between high-flow nasal cannula and conventional oxygen therapy in COVID-19 patients: a systematic review and meta-analysis
Abstract
Background: High-flow nasal cannula (HFNC) and conventional oxygen therapy (COT) are important respiratory support strategies for acute hypoxemic respiratory failure (AHRF) in coronavirus disease 2019 (COVID-19) patients. However, the results are conflicting for the risk of intubation with HFNC as compared to COT.
Objectives: We systematically synthesized the outcomes of HFNC relative to COT in COVID-19 patients with AHRF and evaluated these outcomes in relevant subpopulations.
Design: This study was designed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Data sources and methods: We searched PubMed, EMBASE, Web of Science, Scopus, ClinicalTrials.gov, medRxiv, BioRxiv, and the Cochrane Central Register of Controlled Trials for randomized controlled trials and observational studies that compared the efficacy of HFNC with COT in patients with COVID-19-related AHRF. Primary outcomes were intubation rate and mortality rate. Secondary outcomes were the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2), respiratory rate, hospital length of stay, intensive care unit (ICU) length of stay, and days free from invasive mechanical ventilation.
Results: In total, 20 studies with 5732 patients were included. We found a decreased risk of requiring intubation in HFNC compared to COT [odds ratio (OR) = 0.61, 95% confidence interval (CI): 0.46-0.82, p = 0.0009, I2 = 75%]. Similarly, we found HFNC was associated with lower risk of intubation rate compared to COT in the subgroup of patients with baseline PaO2/FiO2 < 200 mmHg (OR = 0.69, 95% CI: 0.55-0.86, p = 0.0007, I2 = 45%), and who were in ICU settings at enrollment (OR = 0.57, 95% CI: 0.38-0.85, p = 0.005, I2 = 80%). HFNC was associated with an improvement of PaO2/FiO2 and respiratory rate compared to COT. The use of HFNC compared to COT did not reduce the mortality rate, days free from invasive mechanical ventilation, hospital length of stay, or ICU length of stay.
Conclusion: Compared to COT, HFNC may decrease the need for tracheal intubation in patients with COVID-19-related AHRF, particularly among patients with baseline PaO2/FiO2 < 200 mmHg and those in ICU settings.
Trial registration: This systematic review and meta-analysis protocol was prospectively registered with PROSPERO (no. CRD42022339072).
Keywords: COVID-19; conventional oxygen therapy; high-flow nasal cannula.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
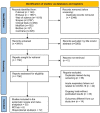

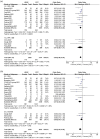

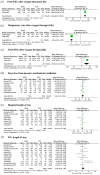
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

