Histone demethylase KDM5 regulates cardiomyocyte maturation by promoting fatty acid oxidation, oxidative phosphorylation, and myofibrillar organization
- PMID: 38230606
- PMCID: PMC11074792
- DOI: 10.1093/cvr/cvae014
Histone demethylase KDM5 regulates cardiomyocyte maturation by promoting fatty acid oxidation, oxidative phosphorylation, and myofibrillar organization
Abstract
Aims: Human pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) provide a platform to identify and characterize factors that regulate the maturation of CMs. The transition from an immature foetal to an adult CM state entails coordinated regulation of the expression of genes involved in myofibril formation and oxidative phosphorylation (OXPHOS) among others. Lysine demethylase 5 (KDM5) specifically demethylates H3K4me1/2/3 and has emerged as potential regulators of expression of genes involved in cardiac development and mitochondrial function. The purpose of this study is to determine the role of KDM5 in iPSC-CM maturation.
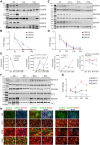
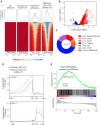
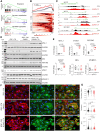
Methods and results: KDM5A, B, and C proteins were mainly expressed in the early post-natal stages, and their expressions were progressively downregulated in the post-natal CMs and were absent in adult hearts and CMs. In contrast, KDM5 proteins were persistently expressed in the iPSC-CMs up to 60 days after the induction of myogenic differentiation, consistent with the immaturity of these cells. Inhibition of KDM5 by KDM5-C70 -a pan-KDM5 inhibitor, induced differential expression of 2372 genes, including upregulation of genes involved in fatty acid oxidation (FAO), OXPHOS, and myogenesis in the iPSC-CMs. Likewise, genome-wide profiling of H3K4me3 binding sites by the cleavage under targets and release using nuclease assay showed enriched of the H3K4me3 peaks at the promoter regions of genes encoding FAO, OXPHOS, and sarcomere proteins. Consistent with the chromatin and gene expression data, KDM5 inhibition increased the expression of multiple sarcomere proteins and enhanced myofibrillar organization. Furthermore, inhibition of KDM5 increased H3K4me3 deposits at the promoter region of the ESRRA gene and increased its RNA and protein levels. Knockdown of ESRRA in KDM5-C70-treated iPSC-CM suppressed expression of a subset of the KDM5 targets. In conjunction with changes in gene expression, KDM5 inhibition increased oxygen consumption rate and contractility in iPSC-CMs.
Conclusion: KDM5 inhibition enhances maturation of iPSC-CMs by epigenetically upregulating the expressions of OXPHOS, FAO, and sarcomere genes and enhancing myofibril organization and mitochondrial function.
Keywords: Epigenetics; H3K4me3; Histone modification; KDM5; iPSC-cardiomyocytes.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared
Figures


Update of
-
Histone demethylase KDM5 regulates cardiomyocyte maturation by promoting fatty acid oxidation, oxidative phosphorylation, and myofibrillar organization.bioRxiv [Preprint]. 2023 May 28:2023.04.11.535169. doi: 10.1101/2023.04.11.535169. bioRxiv. 2023. Update in: Cardiovasc Res. 2024 May 7;120(6):630-643. doi: 10.1093/cvr/cvae014. PMID: 37090524 Free PMC article. Updated. Preprint.
Comment in
-
An emerging epigenetic path towards cardiomyocyte maturation.Cardiovasc Res. 2024 May 7;120(6):563-564. doi: 10.1093/cvr/cvae065. Cardiovasc Res. 2024. PMID: 38537262 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

