Effect of prescription isodose line on tissue sparing in linear accelerator-based stereotactic radiosurgery treating multiple brain metastases using dynamic conformal arcs
- PMID: 38230839
- PMCID: PMC11163483
- DOI: 10.1002/acm2.14278
Effect of prescription isodose line on tissue sparing in linear accelerator-based stereotactic radiosurgery treating multiple brain metastases using dynamic conformal arcs
Abstract
Purpose: Linear accelerator-based stereotactic radiosurgery (SRS) has become a mainstay for simultaneous management of multiple intracranial targets. Recent improvements in treatment planning systems (TPS) have enabled treatment of multiple brain metastases using dynamic conformal arcs (DCA) and a single treatment isocenter. However, as the volume of healthy tissue receiving at least 12 Gy (V12) is linked to the probability of developing radionecrosis, balancing target coverage while minimizing V12 is a critical factor affecting SRS plan quality. Current TPS allow users to adjust various parameters influencing plan optimization. The purpose of this work is to quantify the effect of negative margins on V12 for cranial SRS plans managing multiple brain metastases.
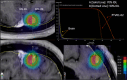
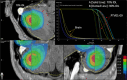
Methods: Using the Brainlab Elements v3.0 TPS (Brainlab, Munich, Germany), we calculated V10, V12, V15, monitor units, and conformity index for seventeen SRS plans treating 2-10 metastases on our Elekta Versa HD (Elekta, Stockholm, Sweden) linear accelerator. We compared plans optimized using 70%-90% prescription isodose lines (IDL) in 5% increments.
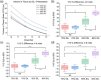
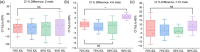
Results: Irrespective of the number of treated metastases, optimization at a lower prescription IDL reduced V10, V12, and V15 and increased MU compared to the 90% IDL (p < 0.01). However, comparing the 70% and 75% IDL optimizations, there was little difference in tissue sparing. The conformity index showed no consistent trends at different IDLs due to a significant spread in case data.
Conclusion: For our plans treating up to 10 metastases, diminishing returns for tissue sparing at IDLs below 80% paired with increasing treatment MU and dosimetric hot spot made optimization at lower IDLs less favorable. In our clinic, after consulting with a physician, it was determined that optimization at the 80% IDL achieved the best balance of V12, treatment MU, and maximum dose. Clinics implementing LINAC-based SRS programs may consider using similar evaluations to develop their own clinical protocols.
Keywords: external beam; stereotactic radiosurgery; treatment planning.
© 2024 The Authors. Journal of Applied Clinical Medical Physics is published by Wiley Periodicals, Inc. on behalf of The American Association of Physicists in Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Direct dosimetric comparison of linear accelerator vs. Gamma Knife fractionated stereotactic radiotherapy (fSRT) of large brain tumors.Med Dosim. 2023 Spring;48(1):31-36. doi: 10.1016/j.meddos.2022.09.006. Epub 2022 Dec 8. Med Dosim. 2023. PMID: 36503990
-
Strategies to optimize stereotactic radiosurgery plans for brain tumors with volumetric-modulated arc therapy.J Appl Clin Med Phys. 2020 Mar;21(3):45-51. doi: 10.1002/acm2.12818. Epub 2020 Feb 11. J Appl Clin Med Phys. 2020. PMID: 32043810 Free PMC article.
-
What is the optimal isodose line for stereotactic radiotherapy for single brain metastases using HyperArc?J Appl Clin Med Phys. 2024 Sep;25(9):e14408. doi: 10.1002/acm2.14408. Epub 2024 Jun 11. J Appl Clin Med Phys. 2024. PMID: 38863310 Free PMC article.
-
Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain.Int J Radiat Oncol Biol Phys. 2021 May 1;110(1):68-86. doi: 10.1016/j.ijrobp.2020.08.013. Epub 2020 Sep 11. Int J Radiat Oncol Biol Phys. 2021. PMID: 32921513 Free PMC article. Review.
-
LINAC based stereotactic radiosurgery for multiple brain metastases: guidance for clinical implementation.Acta Oncol. 2019 Sep;58(9):1275-1282. doi: 10.1080/0284186X.2019.1633016. Epub 2019 Jul 1. Acta Oncol. 2019. PMID: 31257960 Review.
Cited by
-
Consideration of Optimal Evaluation Metrics for Internal Gross Tumor Dose Relevant to Tumor Response in Multi-fraction Stereotactic Radiosurgery of Brain Metastasis.Cureus. 2024 Jul 25;16(7):e65338. doi: 10.7759/cureus.65338. eCollection 2024 Jul. Cureus. 2024. PMID: 39184769 Free PMC article.
-
Impact of High Maximum Dose Constraints Within the Gross Tumor Volume on the Quality of Stereotactic Radiosurgery Plans Using Volumetric-Modulated Arcs for Brain Metastases.Cureus. 2025 Jul 22;17(7):e88548. doi: 10.7759/cureus.88548. eCollection 2025 Jul. Cureus. 2025. PMID: 40851724 Free PMC article.
-
Non-coplanar Arc-Involved Beam Arrangement With Sufficient Arc Rotations Is Suitable for Volumetric-Modulated Arc-Based Radiosurgery for Single Brain Metastasis.Cureus. 2024 Aug 20;16(8):e67265. doi: 10.7759/cureus.67265. eCollection 2024 Aug. Cureus. 2024. PMID: 39301366 Free PMC article.
-
Significance of Inverse Planning With Variable Dose Rate and the Segment Shape Optimization in Dynamic Conformal Arcs Using the High-Definition Dynamic Radiosurgery Platform for Single Brain Metastases.Cureus. 2025 Aug 6;17(8):e89500. doi: 10.7759/cureus.89500. eCollection 2025 Aug. Cureus. 2025. PMID: 40918756 Free PMC article.
References
-
- Chen JC, Rahimian J, Girvigian MR, Miller MJ. Contemporary methods of radiosurgery treatment with the Novalis linear accelerator system. Neurosurg Focus. 2007;23(6):E4. - PubMed
-
- Minniti G, Capone L, Alongi F, et al. Initial experience with single‐isocenter radiosurgery to target multiple brain metastases using an automated treatment planning software: clinical outcomes and optimal target volume margins strategy. Adv Radiat Oncol. 2020;5(5):856‐864. doi:10.1016/j.adro.2020.06.008 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

