Motor patterns of patients with spinal muscular atrophy suggestive of sensory and corticospinal contributions to the development of locomotor muscle synergies
- PMID: 38230872
- PMCID: PMC11321722
- DOI: 10.1152/jn.00513.2022
Motor patterns of patients with spinal muscular atrophy suggestive of sensory and corticospinal contributions to the development of locomotor muscle synergies
Abstract
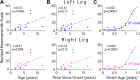
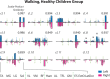
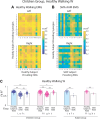
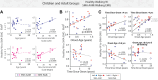
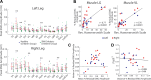
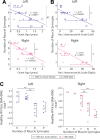
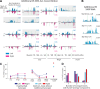
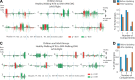
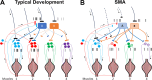
Complex locomotor patterns are generated by combination of muscle synergies. How genetic processes, early sensorimotor experiences, and the developmental dynamics of neuronal circuits contribute to the expression of muscle synergies remains elusive. We shed light on the factors that influence development of muscle synergies by studying subjects with spinal muscular atrophy (SMA, types II/IIIa), a disorder associated with degeneration and deafferentation of motoneurons and possibly motor cortical and cerebellar abnormalities, from which the afflicted would have atypical sensorimotor histories around typical walking onset. Muscle synergies of children with SMA were identified from electromyographic signals recorded during active-assisted leg motions or walking, and compared with those of age-matched controls. We found that the earlier the SMA onset age, the more different the SMA synergies were from the normative. These alterations could not just be explained by the different degrees of uneven motoneuronal losses across muscles. The SMA-specific synergies had activations in muscles from multiple limb compartments, a finding reminiscent of the neonatal synergies of typically developing infants. Overall, while the synergies shared between SMA and control subjects may reflect components of a core modular infrastructure determined early in life, the SMA-specific synergies may be developmentally immature synergies that arise from inadequate activity-dependent interneuronal sculpting due to abnormal sensorimotor experience and other factors. Other mechanisms including SMA-induced intraspinal changes and altered cortical-spinal interactions may also contribute to synergy changes. Our interpretation highlights the roles of the sensory and descending systems to the typical and abnormal development of locomotor modules.NEW & NOTEWORTHY This is likely the first report of locomotor muscle synergies of children with spinal muscular atrophy (SMA), a subject group with atypical developmental sensorimotor experience. We found that the earlier the SMA onset age, the more the subjects' synergies deviated from those of age-matched controls. This result suggests contributions of the sensory/corticospinal activities to the typical expression of locomotor modules, and how their disruptions during a critical period of development may lead to abnormal motor modules.
Keywords: locomotion; motor development; muscle synergy; nonnegative matrix factorization; spinal muscular atrophy.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Exploring the cortical involvement in sensorimotor integration during early stages of independent walking.Exp Brain Res. 2025 May 26;243(6):153. doi: 10.1007/s00221-025-07099-4. Exp Brain Res. 2025. PMID: 40418430 Free PMC article.
-
Motor module activation sequence and topography in the spinal cord during air-stepping in human: Insights into the traveling wave in spinal locomotor circuits.Physiol Rep. 2017 Nov;5(22):e13504. doi: 10.14814/phy2.13504. Physiol Rep. 2017. PMID: 29180480 Free PMC article.
-
Common and distinct muscle synergies during level and slope locomotion in the cat.J Neurophysiol. 2021 Aug 1;126(2):493-515. doi: 10.1152/jn.00310.2020. Epub 2021 Jun 30. J Neurophysiol. 2021. PMID: 34191619 Free PMC article.
-
Representation of Muscle Synergies in the Primate Brain.J Neurosci. 2015 Sep 16;35(37):12615-24. doi: 10.1523/JNEUROSCI.4302-14.2015. J Neurosci. 2015. PMID: 26377453 Free PMC article. Review.
-
Brain Magnetic Resonance Imaging (MRI) in Spinal Muscular Atrophy: A Scoping Review.J Neuromuscul Dis. 2023;10(4):493-503. doi: 10.3233/JND-221567. J Neuromuscul Dis. 2023. PMID: 37125560 Free PMC article.
Cited by
-
State-dependent neural representations of muscle synergies in the spinal cord revealed by optogenetic stimulation.J Physiol. 2025 Aug;603(16):4659-4679. doi: 10.1113/JP288073. Epub 2025 Jul 28. J Physiol. 2025. PMID: 40726105 Free PMC article.
-
Motor synergy and energy efficiency emerge in whole-body locomotion learning.Sci Rep. 2025 Jan 3;15(1):712. doi: 10.1038/s41598-024-82472-x. Sci Rep. 2025. PMID: 39753645 Free PMC article.
-
Rectified Latent Variable Model-Based EMG Factorization of Inhibitory Muscle Synergy Components Related to Aging, Expertise and Force-Tempo Variations.Sensors (Basel). 2024 Apr 28;24(9):2820. doi: 10.3390/s24092820. Sensors (Basel). 2024. PMID: 38732926 Free PMC article.
-
Exploring the cortical involvement in sensorimotor integration during early stages of independent walking.Exp Brain Res. 2025 May 26;243(6):153. doi: 10.1007/s00221-025-07099-4. Exp Brain Res. 2025. PMID: 40418430 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

