Longitudinal gut microbiota composition of South African and Nigerian infants in relation to tetanus vaccine responses
- PMID: 38230936
- PMCID: PMC10846250
- DOI: 10.1128/spectrum.03190-23
Longitudinal gut microbiota composition of South African and Nigerian infants in relation to tetanus vaccine responses
Abstract
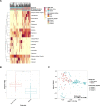
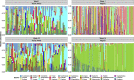
Infants who are exposed to HIV but uninfected (iHEU) have higher risk of infectious morbidity than infants who are HIV-unexposed and uninfected (iHUU), possibly due to altered immunity. As infant gut microbiota may influence immune development, we evaluated the effects of HIV exposure on infant gut microbiota and its association with tetanus toxoid vaccine responses. We evaluated the gut microbiota of 82 South African (61 iHEU and 21 iHUU) and 196 Nigerian (141 iHEU and 55 iHUU) infants at <1 and 15 weeks of life by 16S rRNA gene sequencing. Anti-tetanus antibodies were measured by enzyme-linked immunosorbent assay at matched time points. Gut microbiota in the 278 included infants and its succession were more strongly influenced by geographical location and age than by HIV exposure. Microbiota of Nigerian infants, who were exclusively breastfed, drastically changed over 15 weeks, becoming dominated by Bifidobacterium longum subspecies infantis. This change was not observed among South African infants, even when limiting the analysis to exclusively breastfed infants. The Least Absolute Shrinkage and Selection Operator regression suggested that HIV exposure and gut microbiota were independently associated with tetanus titers at week 15, and that high passively transferred antibody levels, as seen in the Nigerian cohort, may mitigate these effects. In conclusion, in two African cohorts, HIV exposure minimally altered the infant gut microbiota compared to age and setting, but both specific gut microbes and HIV exposure independently predicted humoral tetanus vaccine responses.IMPORTANCEGut microbiota plays an essential role in immune system development. Since infants HIV-exposed and uninfected (iHEU) are more vulnerable to infectious diseases than unexposed infants, we explored the impact of HIV exposure on gut microbiota and its association with vaccine responses. This study was conducted in two African countries with rapidly increasing numbers of iHEU. Infant HIV exposure did not substantially affect gut microbial succession, but geographic location had a strong effect. However, both the relative abundance of specific gut microbes and HIV exposure were independently associated with tetanus titers, which were also influenced by baseline tetanus titers (maternal transfer). Our findings provide insight into the effect of HIV exposure, passive maternal antibody, and gut microbiota on infant humoral vaccine responses.
Keywords: HIV-exposed uninfected infants; Nigeria; South Africa; gut microbiota; tetanus toxoid; vaccine response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Update of
-
Longitudinal gut microbiota composition of South African and Nigerian infants in relation to tetanus vaccine responses.Res Sq [Preprint]. 2023 Jul 3:rs.3.rs-3112263. doi: 10.21203/rs.3.rs-3112263/v1. Res Sq. 2023. Update in: Microbiol Spectr. 2024 Feb 6;12(2):e0319023. doi: 10.1128/spectrum.03190-23. PMID: 37461449 Free PMC article. Updated. Preprint.
Similar articles
-
Longitudinal gut microbiota composition of South African and Nigerian infants in relation to tetanus vaccine responses.Res Sq [Preprint]. 2023 Jul 3:rs.3.rs-3112263. doi: 10.21203/rs.3.rs-3112263/v1. Res Sq. 2023. Update in: Microbiol Spectr. 2024 Feb 6;12(2):e0319023. doi: 10.1128/spectrum.03190-23. PMID: 37461449 Free PMC article. Updated. Preprint.
-
Bifidobacterium infantis supplementation versus placebo in early life to improve immunity in infants exposed to HIV: a protocol for a randomized trial.BMC Complement Med Ther. 2023 Oct 18;23(1):367. doi: 10.1186/s12906-023-04208-0. BMC Complement Med Ther. 2023. PMID: 37853370 Free PMC article.
-
Evolution of the Gut Microbiome in HIV-Exposed Uninfected and Unexposed Infants during the First Year of Life.mBio. 2022 Oct 26;13(5):e0122922. doi: 10.1128/mbio.01229-22. Epub 2022 Sep 8. mBio. 2022. PMID: 36073815 Free PMC article.
-
The Effect of Human Immunodeficiency Virus and Cytomegalovirus Infection on Infant Responses to Vaccines: A Review.Front Immunol. 2018 Mar 2;9:328. doi: 10.3389/fimmu.2018.00328. eCollection 2018. Front Immunol. 2018. PMID: 29552009 Free PMC article. Review.
-
Impact of maternal HIV exposure, feeding status, and microbiome on infant cellular immunity.J Leukoc Biol. 2019 Feb;105(2):281-289. doi: 10.1002/JLB.MR0318-120R. Epub 2018 Dec 21. J Leukoc Biol. 2019. PMID: 30577072 Free PMC article. Review.
Cited by
-
Neonates exposed to HIV but uninfected exhibit an altered gut microbiota and inflammation associated with impaired breast milk antibody function.Microbiome. 2024 Dec 20;12(1):261. doi: 10.1186/s40168-024-01973-z. Microbiome. 2024. PMID: 39707483 Free PMC article.
References
-
- Cohen C, Moyes J, Tempia S, Groome M, Walaza S, Pretorius M, Naby F, Mekgoe O, Kahn K, von Gottberg A, Wolter N, Cohen AL, von Mollendorf C, Venter M, Madhi SA. 2016. Epidemiology of acute lower respiratory tract infection in HIV-exposed uninfected infants. Pediatrics 137:e20153272. doi:10.1542/peds.2015-3272 - DOI - PMC - PubMed
-
- Smith C, Moraka NO, Ibrahim M, Moyo S, Mayondi G, Kammerer B, Leidner J, Gaseitsiwe S, Li S, Shapiro R, Lockman S, Weinberg A. 2020. Human immunodeficiency virus exposure but not early cytomegalovirus infection is associated with increased hospitalization and decreased memory T-cell responses to tetanus vaccine. J Infect Dis 221:1167–1175. doi:10.1093/infdis/jiz590 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

