Prednisone vs high-dose dexamethasone in newly diagnosed adult primary immune thrombocytopenia: a randomized trial
- PMID: 38231017
- PMCID: PMC10966179
- DOI: 10.1182/bloodadvances.2023010975
Prednisone vs high-dose dexamethasone in newly diagnosed adult primary immune thrombocytopenia: a randomized trial
Abstract
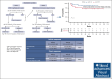
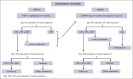
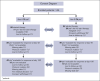
A debate exists regarding which type of corticosteroids (standard-dose prednisone [PDN] or high-dose dexamethasone [HD-DXM]) is the best first-line treatment for adult patients with newly diagnosed untreated primary immune thrombocytopenia (pITP). An ad hoc study compared PDN with HD-DXM in newly diagnosed untreated patients with pITP (aged ≥18 but ≤80 years, platelet count of ≤20 or >20 but <50 × 109/L, and bleeding score of ≥8). Patients were randomised to receive PDN 1 mg/kg per day from days 0 to 28 (Arm A) or HD-DXM 40 mg per day for 4 days, every 14 days, for 3 consecutive courses (Arm B). Fifty-nine of 113 patients (52.2%) were randomized to Arm A and 54 of 113 (47.8%) to Arm B. In evaluable patients, total initial responses (complete response [CR], partial response [PR], minimal response [MR]) were 44 of 56 (78.57%) in Arm A and 46 of 49 (93.88%) in Arm B at days 42 and 46, respectively (P = 0.0284). Total final responses (at day 180 from initial response) were 26 of 43 (60.47%) in Arm A and 23 of 39 (58.97%) in Arm B (P = 0.8907). Total persistent responses (at 12 months from initial response) were 25 of 31 (80.65%) in Arm A and 20 of 36 (55.56%) in Arm B (P = 0.0292). Seven relapses occurred. Median follow-up was 44.4 months. Overall survival was 100% at 48 months, overall disease-free survival was 81.11% at 48 months from day 180. PDN and pulsed HD-DXM were well tolerated; HD-DXM allows effective initial responses but less long lasting than PDN. This trial was registered at www.clinicaltrials.gov as #NCT00657410.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.S. participated in advisory boards for Amgen, Novartis, and Sobi, and was a member of the speakers' bureaus for Amgen, Novartis, and Sobi. E.B. participated in advisory boards for Amgen and Novartis. F.P. received honoraria from Novartis and Sobi. E. Rivolti participated in advisory boards for Novartis and Sobi, and reports presentations for Novartis and Amgen. A.P. was a consultant for Sanofi, Alexion, and Takeda; reports presentations for Sobi, Novartis, Pfizer, and Sanofi; and received congress fees from Alexion and Takeda. M.D. participated in advisory boards for Amgen and Novartis. S. Siragus collaborated with Sobi, Bayer, Amgen, Novartis, and Novo Nordisk. V.D.S. participated in advisory boards for Argenx, Grifols, Novartis, and Sobi, and was a member of the speakers' bureaus for Amgen, Grifols, and Novartis. E. Rossi participated in advisory boards for Argenx, Grifols, Novartis, Amgen, and Sobi, and was a member of the speakers' bureaus for Amgen, Novartis, and Sobi. F.Z. received personal fees from Novartis, Amgen, Roche, AbbVie, Janssen Cilag, Takeda, Grifols, Argenx, Sobi, and BeiGene; received funding for biologic studies or clinical trials from Novartis, Amgen, AbbVie, and Janssen Cilag; and received fundings for educational projects from Janssen Cilag, AbbVie, Kyowa Kirin, Takeda, BeiGene, and AstraZeneca. M.B. received conference fees from Incyte, AstraZeneca, Novartis, and Janssen. M.C. was a member of the speakers' bureaus for Novartis and Bristol Myers Squibb. A.T. received congress fees from Novartis and research funds from Janssen. N.V. participated in advisory boards and was speaker for Novartis, Amgen, Grifols, and Sobi. The remaining authors declare no competing financial interests.
Figures
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an International Working Group. Blood. 2009;113(11):2386–2393. - PubMed
-
- Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145(2):235–244. - PubMed
-
- Frederiksen H, Schmidt K. The incidence of idiopathic thrombocytopenic purpura in adults increases with age. Blood. 1999;94(3):909–913. - PubMed
-
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. - PubMed
-
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr., Crowther MA, American Society of Hematology The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials