Evolution of the gene regulatory network of body axis by enhancer hijacking in amphioxus
- PMID: 38231024
- PMCID: PMC10794066
- DOI: 10.7554/eLife.89615
Evolution of the gene regulatory network of body axis by enhancer hijacking in amphioxus
Abstract
A central goal of evolutionary developmental biology is to decipher the evolutionary pattern of gene regulatory networks (GRNs) that control embryonic development, and the mechanism underlying GRNs evolution. The Nodal signaling that governs the body axes of deuterostomes exhibits a conserved GRN orchestrated principally by Nodal, Gdf1/3, and Lefty. Here we show that this GRN has been rewired in cephalochordate amphioxus. We found that while the amphioxus Gdf1/3 ortholog exhibited nearly no embryonic expression, its duplicate Gdf1/3-like, linked to Lefty, was zygotically expressed in a similar pattern as Lefty. Consistent with this, while Gdf1/3-like mutants showed defects in axial development, Gdf1/3 mutants did not. Further transgenic analyses showed that the intergenic region between Gdf1/3-like and Lefty could drive reporter gene expression as that of the two genes. These results indicated that Gdf1/3-like has taken over the axial development role of Gdf1/3 in amphioxus, possibly through hijacking Lefty enhancers. We finally demonstrated that, to compensate for the loss of maternal Gdf1/3 expression, Nodal has become an indispensable maternal factor in amphioxus and its maternal mutants caused axial defects as Gdf1/3-like mutants. We therefore demonstrated a case that the evolution of GRNs could be triggered by enhancer hijacking events. This pivotal event has allowed the emergence of a new GRN in extant amphioxus, presumably through a stepwise process. In addition, the co-expression of Gdf1/3-like and Lefty achieved by a shared regulatory region may have provided robustness during body axis formation, which provides a selection-based hypothesis for the phenomena called developmental system drift.
Keywords: Nodal signaling; amphioxus; body axis; enhancer hijacking; evolutionary biology; gene regulatory network.
© 2024, Shi et al.
Conflict of interest statement
CS, SC, HL, RP, SL, YW, XW, JL, XL, CX, XL, YW, QQ, GL No competing interests declared
Figures


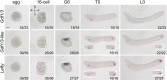
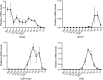
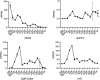

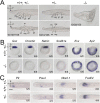











Update of
- doi: 10.1101/2023.06.11.544506
References
-
- Brasó-Vives M, Marlétaz F, Echchiki A, Mantica F, Acemel RD, Gómez-Skarmeta JL, Hartasánchez DA, Le Targa L, Pontarotti P, Tena JJ, Maeso I, Escriva H, Irimia M, Robinson-Rechavi M. Parallel evolution of amphioxus and vertebrate small-scale gene duplications. Genome Biology. 2022;23:243. doi: 10.1186/s13059-022-02808-6. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- SRA/PRJNA603159
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

