Onvansertib in Combination with FOLFIRI and Bevacizumab in Second-Line Treatment of KRAS-Mutant Metastatic Colorectal Cancer: A Phase Ib Clinical Study
- PMID: 38231047
- PMCID: PMC11094418
- DOI: 10.1158/1078-0432.CCR-23-3053
Onvansertib in Combination with FOLFIRI and Bevacizumab in Second-Line Treatment of KRAS-Mutant Metastatic Colorectal Cancer: A Phase Ib Clinical Study
Abstract
Purpose: Onvansertib is a highly specific inhibitor of polo-like kinase 1 (PLK1), with demonstrated safety in solid tumors. We evaluated, preclinically and clinically, the potential of onvansertib in combination with chemotherapy as a therapeutic option for KRAS-mutant colorectal cancer.
Patients and methods: Preclinical activity of onvansertib was assessed (i) in vitro in KRAS wild-type and -mutant isogenic colorectal cancer cells and (ii) in vivo, in combination with irinotecan, in a KRAS-mutant xenograft model. Clinically, a phase Ib trial was conducted to investigate onvansertib at doses 12, 15, and 18 mg/m2 (days 1-5 and 14-19 of a 28-day cycle) in combination with FOLFIRI/bevacizumab (days 1 and 15) in patients with KRAS-mutant metastatic colorectal cancer who had prior oxaliplatin exposure. Safety, efficacy, and changes in circulating tumor DNA (ctDNA) were assessed.
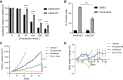
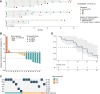
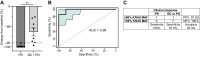
Results: In preclinical models, onvansertib displayed superior activity in KRAS-mutant than wild-type isogenic colorectal cancer cells and demonstrated potent antitumor activity in combination with irinotecan in vivo. Eighteen patients enrolled in the phase Ib study. Onvansertib recommended phase II dose was established at 15 mg/m2. Grade 3 and 4 adverse events (AE) represented 15% of all treatment-related AEs, with neutropenia being the most common. Partial responses were observed in 44% of patients, with a median duration of response of 9.5 months. Early ctDNA dynamics were predictive of treatment efficacy.
Conclusions: Onvansertib combined with FOLIFRI/bevacizumab exhibited manageable safety and promising efficacy in second-line treatment of patients with KRAS-mutant metastatic colorectal cancer. Further exploration of this combination therapy is ongoing. See related commentary by Stebbing and Bullock, p. 2005.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures
Comment in
-
Polo-like Kinase 1 Inhibition in KRAS-Mutated Metastatic Colorectal Cancer.Clin Cancer Res. 2024 May 15;30(10):2005-2007. doi: 10.1158/1078-0432.CCR-24-0251. Clin Cancer Res. 2024. PMID: 38470499
-
PLK1 and its role in the evolving landscape of KRAS-mutated colorectal cancer.J Gastrointest Oncol. 2024 Aug 31;15(4):1987-1992. doi: 10.21037/jgo-24-323. Epub 2024 Aug 19. J Gastrointest Oncol. 2024. PMID: 39279930 Free PMC article. No abstract available.
References
-
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen Y-J, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:329–59. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. - PubMed
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337–45. - PubMed
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55. - PubMed
-
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

