Guadecitabine vs TC in relapsed/refractory AML after intensive chemotherapy: a randomized phase 3 ASTRAL-2 trial
- PMID: 38231126
- PMCID: PMC11103175
- DOI: 10.1182/bloodadvances.2023012062
Guadecitabine vs TC in relapsed/refractory AML after intensive chemotherapy: a randomized phase 3 ASTRAL-2 trial
Abstract
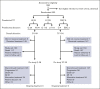
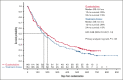
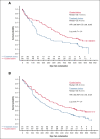
Guadecitabine is a novel hypomethylating agent (HMA) resistant to deamination by cytidine deaminase. Patients with relapsed/refractory acute myeloid leukemia (AML) were randomly assigned to guadecitabine or a preselected treatment choice (TC) of high-intensity chemotherapy, low-intensity treatment with HMAs or low-dose cytarabine, or best supportive care (BSC). The primary end point was overall survival (OS). A total of 302 patients were randomly assigned to guadecitabine (n = 148) or TC (n = 154). Preselected TCs were low-intensity treatment (n = 233 [77%; mainly HMAs]), high-intensity chemotherapy (n = 63 [21%]), and BSC (n = 6 [2%]). The median OS were 6.4 and 5.4 months for guadecitabine and TC, respectively (hazard ratio 0.88 [95% confidence interval, 0.67-1.14]; log-rank P = .33). Survival benefit for guadecitabine was suggested in several prospective subgroups, including age <65 years, Eastern Cooperative Oncology Group performance status 0 to 1, refractory AML, and lower peripheral blood blasts ≤30%. Complete response (CR) + CR with partial hematologic recovery rates were 17% for guadecitabine vs 8% for TC (P < .01); CR+CR with incomplete count recovery rates were 27% for guadecitabine vs 14% for TC (P < .01). Safety was comparable for the 2 arms, but guadecitabine had a higher rate of grade ≥3 neutropenia (32% vs 17%; P < .01). This study did not demonstrate an OS benefit for guadecitabine. Clinical response rates were higher for guadecitabine, with comparable safety to TC. There was an OS benefit for guadecitabine in several prespecified subgroups. This study was registered at www.clinicaltrials.gov as #NCT02920008.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: G.J.R. reports consultancy for AbbVie, Amgen, Argenx, AstraZeneca, bluebird bio, Blueprint, Bristol Myers Squibb (BMS), Caribou, Celgene, Daiichi Sankyo, Ellipses, GlaxoSmithKline (GSK), Janssen, Jasper, Jazz, Molecular Partners, Novartis, Pfizer, Rigel, Roche, Syndax, Takeda, and Telix, and received research support from Janssen. G.S. received honoraria, has advisory board membership, and received consultation fees or travel expenses from AbbVie, AstraZeneca, BeiGene, BMS, ExCellThera, Novartis, Roche, and Takeda. E.A.G received study support to institution (Roswell Park) and writing support from Astex; received research support to Roswell Park from Alexion, Apellis, Astex, Blueprint, BMS, Celdex, and Genentech; is on the advisory boards of AbbVie, Alexion/AZ, Apellis, BMS, CTI Biopharma, Novartis, Partner Therapeutics, Taiho, and Takeda; received payment/honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Aplastic Anemia and Myelodysplastic Syndrome International Foundation, American Society of Hematology, MedScape, Karger Publishing, MediCom Worldwide, and Physicians Educational Resource; received support for attending meetings/travel from MDS International; is on the advisory boards of Dresner Foundation and Picnic Health; and has leadership/fiduciary role in other board, society, committee, or advocacy group of Dresner, National Comprehensive Cancer Network Guidelines, and Via Pathways-Elsevier. K.Y. reports consultancy for BMS/Celgene, GSK, Jazz, Novartis, Pfizer, Roche, Shattuck, Taiho, and Takeda, and received research funding from Astex, Forma, Genentech, Geron, Gilead, Janssen, Jazz, Novartis, Roche, and Treadwell; received honoraria from AbbVie, Novartis, and Taiho. H.K. reports honoraria/advisory board/consulting from/at AbbVie, Amgen, Amphista, Ascentage, Astellas, Biologix, Curis, Ipsen, KAHR Medical, Labcorp, Novartis, Pfizer, Shenzhen Target Rx, Stemline, and Takeda, and received research grants from AbbVie, Amgen, Ascentage, BMS, Daiichi Sankyo, ImmunoGen, Jazz, and Novartis. C.R. received research funding from AbbVie, Amgen, Astellas, BMS, Iqvia, and Jazz; received payment/honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events for AbbVie, Amgen, Astellas, Jazz, Novartis, and Servier; received support for attending meetings/travel from AbbVie and Servier; and is on the advisory boards of AbbVie, Amgen, Astellas, BMS, Boehringer, Jazz, and Servier. M.T.B. received research funding from Karyopharm. E.P. received consulting fees from KCR US; payment/honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Amgen, Angelini, Astellas, Novartis, Servier; and support for attending meetings/travel from Angelini, Astellas, BMS, Jazz, Novartis, Pfizer, and Servier. H.-J.K. received study grant from BL & H; consulting fees from AbbVie, AIMS, Amgen, AML-Hub, Astellas, Aston, BMS/Celgene, Boryung, Daiichi Sankyo, GreenCross, Handkok, Ingenium, Janssen, LG Chem, Meiji, Novartis, Pfizer, Sanofi, SL VaxiGen, Takeda, and VigenCell; payment/honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from AbbVie, AML-Hub, Astellas, BMS, Handok, Novartis; is on the data safety monitoring/advisory board of AbbVie, AML-Hub, Astellas, BMS, Daiichi Sankyo, Handok, Janssen, Novartis, Pfixer, and Sanofi; and has a leadership/fiduciary role in other board, society, committee, or advocacy group of AML-Hub, Asia-Pacific Blood and Marrow Transplantation Group, APLC, BMS, International Congress of Bone Marrow Transplantation, and Novartis. A.I. received consulting fees from Celgene, Janssen, Novartis, Pfizer, Roche, and Takeda, and support for attending meetings/travel from Janssen, Novartis, Pfizer, and Roche. P.F. received research support from Astex. Y.M. received honoraria from AbbVie, Astellas, BMS, Chugai, Daiichi Sankyo, Eisai, Janssen, Kyowa-Kirin, Nippon-Shinyaku, Novartis, Pfizer, Sanofi, Sumitomo-Dainippon, and Takeda, and received research funding from Sumitomo-Dainippon. T.Y. received research funding from AbbVie, Daiichi Sankyo, Otsuka, Pfizer, and Solasia, and received honoraria from Pfizer. C.L.O. received research support from Astex and Genentech. Y.H., H.N.K., and M.A. are employees of Astex. H.D. reports consultancy for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, BMS, Celgene, Daiichi Sankyo, Gilead, Janssen, Jazz, Novartis, Servier, Stemline, and Syndax, and clinical research funding to Ulm University Hospital from AbbVie, Agios, Amgen, Astellas, BMS, Celgene, Jazz, Kronos, Novartis, and Pfizer. The remaining authors have no compting financial interests to declare.
Figures


Comment in
-
Survival for the fittest: guadecitabine in rel/ref AML.Blood Adv. 2024 Apr 23;8(8):2018-2019. doi: 10.1182/bloodadvances.2024012569. Blood Adv. 2024. PMID: 38652486 Free PMC article. No abstract available.
References
-
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. - PubMed
-
- Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase iii study of elacytarabine versus investigator choice in patients with acute myeloid leukemia. J Clin Oncol. 2014;32(18):1919–1926. - PubMed

