Uptake of Cyclodextrin Nanoparticles by Macrophages is Dependent on Particle Size and Receptor-Mediated Interactions
- PMID: 38231485
- PMCID: PMC11252246
- DOI: 10.1021/acsabm.3c00985
Uptake of Cyclodextrin Nanoparticles by Macrophages is Dependent on Particle Size and Receptor-Mediated Interactions
Abstract
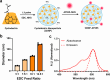
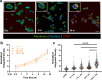
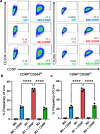
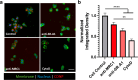
Physiochemical properties of nanoparticles, such as their size and chemical composition, dictate their interaction with professional phagocytes of the innate immune system. Macrophages, in particular, are key regulators of the immune microenvironment that heavily influence particle biodistribution as a result of their uptake. This attribute enables macrophage-targeted delivery, including for phenotypic modulation. Saccharide-based materials, including polyglucose polymers and nanoparticles, are efficient vehicles for macrophage-targeted delivery. Here, we investigate the influence of particle size on cyclodextrin nanoparticle (CDNP) uptake by macrophages and further examine the receptor-mediated interactions that drive macrophage-targeted delivery. We designed and synthesized CDNPs ranging in size from 25 nm to >100 nm in diameter. Increasing particle size was correlated with greater uptake by macrophages in vitro. Both scavenger receptor A1 and mannose receptor were critical mediators of macrophage-targeted delivery, inhibition of which reduced the extent of uptake. Finally, we investigated the cellular bioavailability of drug-loaded CDNPs using a model anti-inflammatory drug, celastrol, which demonstrated that drug bioactivity is improved by CDNP loading relative to free drug alone. This study thus elucidates the interactions between the polyglucose nanoparticles and macrophages, thereby facilitating their application in macrophage-targeted drug delivery that has applications in the context of tissue injury and repair.
Keywords: Nanomedicine; cyclodextrin; macrophage; nanoparticle; receptor-mediated uptake.
Conflict of interest statement
The authors declare the following competing financial interest(s): C.B.R. is listed on a patent filed by Partners Healthcare pertaining to the development of the CDNP. The remaining authors declare no competing interests.
Figures

Similar articles
-
Mannosyl-coated nanocomplexes from amphiphilic cyclodextrins and pDNA for site-specific gene delivery.Biomaterials. 2011 Oct;32(29):7263-73. doi: 10.1016/j.biomaterials.2011.06.025. Epub 2011 Jul 7. Biomaterials. 2011. PMID: 21741082
-
M3-DPPE Liposomal Nanoparticles Encapsulating CLEC12A Enhance CD206-Mediated Endocytosis and Efficacy in the Collagen-Induced Arthritis Model.ACS Appl Bio Mater. 2025 Feb 17;8(2):1002-1016. doi: 10.1021/acsabm.4c01139. Epub 2025 Jan 10. ACS Appl Bio Mater. 2025. PMID: 39794898
-
In Vivo siRNA Delivery to Immunosuppressive Liver Macrophages by α-Mannosyl-Functionalized Cationic Nanohydrogel Particles.Cells. 2020 Aug 15;9(8):1905. doi: 10.3390/cells9081905. Cells. 2020. PMID: 32824208 Free PMC article.
-
Redox biology of Leishmania and macrophage targeted nanoparticles for therapy.Nanomedicine (Lond). 2017 Jul;12(14):1713-1725. doi: 10.2217/nnm-2017-0049. Epub 2017 Jun 21. Nanomedicine (Lond). 2017. PMID: 28635366 Review.
-
Current status of mannose receptor-targeted drug delivery for improved anti-HIV therapy.J Control Release. 2024 Aug;372:494-521. doi: 10.1016/j.jconrel.2024.06.002. Epub 2024 Jun 27. J Control Release. 2024. PMID: 38849091 Review.
Cited by
-
Investigation of Factors Influencing the Effectiveness of Deformable Nanovesicles for Insulin Nebulization Inhalation.Pharmaceutics. 2024 Jun 29;16(7):879. doi: 10.3390/pharmaceutics16070879. Pharmaceutics. 2024. PMID: 39065576 Free PMC article.
References
-
- Bill R.; Wirapati P.; Messemaker M.; Roh W.; Zitti B.; Duval F.; Kiss M.; Park J. C.; Saal T. M.; Hoelzl J.; Tarussio D.; Benedetti F.; Tissot S.; Kandalaft L.; Varrone M.; Ciriello G.; McKee T. A.; Monnier Y.; Mermod M.; Blaum E. M.; Gushterova I.; Gonye A. L. K.; Hacohen N.; Getz G.; Mempel T. R.; Klein A. M.; Weissleder R.; Faquin W. C.; Sadow P. M.; Lin D.; Pai S. I.; Sade-Feldman M.; Pittet M. J. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science 2023, 381 (6657), 515–524. 10.1126/science.ade2292. - DOI - PMC - PubMed
-
- Rodell C. B.; Arlauckas S. P.; Cuccarese M. F.; Garris C. S.; Li R.; Ahmed M. S.; Kohler R. H.; Pittet M. J.; Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed Eng. 2018, 2 (8), 578–588. 10.1038/s41551-018-0236-8. - DOI - PMC - PubMed
-
- Turco V.; Pfleiderer K.; Hunger J.; Horvat N. K.; Karimian-Jazi K.; Schregel K.; Fischer M.; Brugnara G.; Jahne K.; Sturm V.; Streibel Y.; Nguyen D.; Altamura S.; Agardy D. A.; Soni S. S.; Alsasa A.; Bunse T.; Schlesner M.; Muckenthaler M. U.; Weissleder R.; Wick W.; Heiland S.; Vollmuth P.; Bendszus M.; Rodell C. B.; Breckwoldt M. O.; Platten M. T cell-independent eradication of experimental glioma by intravenous TLR7/8-agonist-loaded nanoparticles. Nat. Commun. 2023, 14 (1), 771.10.1038/s41467-023-36321-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
