Urinary CD4 + T Cells Predict Renal Relapse in ANCA-Associated Vasculitis
- PMID: 38231590
- PMCID: PMC11000730
- DOI: 10.1681/ASN.0000000000000311
Urinary CD4 + T Cells Predict Renal Relapse in ANCA-Associated Vasculitis
Abstract
Significance statement: Early identification of patients at risk of renal flares in ANCA vasculitis is crucial. However, current clinical parameters have limitations in predicting renal relapse accurately. This study investigated the use of urinary CD4 + T lymphocytes as a predictive biomarker for renal flares in ANCA vasculitis. This study, including urine samples from 102 patients, found that the presence of urinary CD4 + T cells was a robust predictor of renal relapse within a 6-month time frame, with a sensitivity of 60% and a specificity of 97.8%. The diagnostic accuracy of urinary CD4 + T cells exceeded that of ANCA titers, proteinuria, and hematuria. Monitoring urinary CD4 + T lymphocytes could help assess the risk of future renal relapse, enabling early preventive measures and tailored treatment strategies.
Background: In ANCA-associated vasculitis, there is a lack of biomarkers for predicting renal relapse. Urinary T cells have been shown to differentiate active GN from remission in ANCA-associated vasculitis, but their predictive value for renal flares remains unknown.
Methods: The PRE-FLARED study was a prospective multicenter biomarker study including 102 individuals with ANCA-associated vasculitis in remission aimed to predict renal relapse by quantifying urinary CD4 + T-cell subsets using flow cytometry at baseline and monitoring clinical outcomes over a 6-month follow-up.
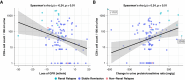
Results: Among the participants, ten experienced renal relapses, two had non-renal flares, and 90 remained in stable remission. The median baseline urinary CD4 + T-cell count was significantly higher in patients who relapsed compared with those in remission. Receiver operating characteristic curve analysis of urinary CD4 + T-cell counts showed an area under the curve value of 0.88 for predicting renal flares, outperforming ANCA titers, hematuria, and proteinuria. Using a cutoff of 490 CD4 + T cells per 100 ml urine, the sensitivity and specificity in identifying patients with future renal flares were 60% and 97.8%, respectively. In a post hoc analysis, combining urinary CD4 + T-cell counts with proteinase-3 ANCA levels suggested improved predictive performance in the PR3 + subgroup. In addition, the number of urinary CD4 + T cells showed a limited correlation with a decline in GFR and an increase in proteinuria over the follow-up period.
Conclusions: This study concluded that urinary CD4 + T-cell counts could identify patients with ANCA-associated vasculitis at a substantial risk of renal relapse within 6 months. Combining these counts with ANCA levels further improved the prediction of relapse.
Clinical trial registry name and registration number: Urinary T Lymphocytes Predict Renal Flares in Patients With Inactive ANCA-associated Glomerulonephritis (PRE-FLARED), NCT04428398 .
Copyright © 2024 by the American Society of Nephrology.
Conflict of interest statement
M. Bieringer reports employment with Helios Klinikum Berlin-Buch and speakers bureau for Vifor Pharma Deutschland GmbH. K.-U. Eckardt reports employment with Charité - Universitätsmedizin Berlin; consultancy for Akebia, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, and Otsuka; research funding from Bayer, Evotec, and Travere; honoraria from Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, GSK, Novartis, Sanofi, and Otsuka; and role on the Editorial Boards of
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

