Effects of Daily Ingestion of Two SunGold Kiwifruit for 6 Weeks on Metabolic and Inflammatory Biomarkers: A Randomized, Cross-Over, Exploratory Intervention Study
- PMID: 38231672
- PMCID: PMC10705898
- DOI: 10.3390/foods12234236
Effects of Daily Ingestion of Two SunGold Kiwifruit for 6 Weeks on Metabolic and Inflammatory Biomarkers: A Randomized, Cross-Over, Exploratory Intervention Study
Abstract
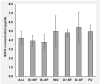
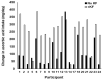
Kiwifruit contain many components, some considered beneficial, such as vitamins, phytochemicals and dietary fibre, and others potentially harmful, such as fructose and glucose in fruit sugars. In a 6-week, randomised, crossover study aimed at exploring the net effects of daily consumption of kiwifruit, 23 healthy participants consumed two Actinidia chinensis var. chinensis 'Zesy002' (marketed as Zespri™ SunGold™ Kiwifruit) per day as part of their customary diet (intervention) or without kiwifruit (control) as their customary diet for 6 weeks in a cross-over study. Anthropometric data, venous blood, and urine samples were collected at the start and end of the 6-week intervention and control periods for the measurement of physical changes, plasma glucose, insulin, glycated haemoglobin, short-chain fatty acids, blood lipids, uric acid, inflammatory biomarkers, and urinary ascorbic acid. Variables were measured between the start and finish of interventions, and between intervention and control periods. Food diaries were completed on the 3 days before blood sampling to estimate dietary ascorbic acid and dietary fibre intakes. Despite urinary vitamin C and food diaries indicating compliance, and good precision in measurements, there were no appreciable changes in biomarkers during the study, either within or between intervention and control periods, that would indicate a change in health status. Thus, the sizes of any effects of kiwifruit ingestion were too small to become significant under the test conditions used, indicating a high probability that daily ingestion of two SunGold kiwifruit is safe with respect to metabolic health.
Keywords: C-reactive protein; inflammation; kiwifruit; metabolic health; plasma lipids; plasma short-chain fatty acids; vitamin C.
Conflict of interest statement
The authors declare no conflicts of interest. The study was funded internally from the Kiwifruit Royalty Investment Program (KRIP), but the kiwifruit industry had no role in the design of the study, in the collection, analysis, or interpretation of data, or in the writing of the manuscript.
Figures





References
-
- Wang D.D., Li Y.P., Bhupathiraju S.N., Rosner B.A., Sun Q., Giovannucci E.L., Rimm E.B., Manson J.E., Willett W.C., Stampfer M.J., et al. Fruit and Vegetable Intake and Mortality Results from 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation. 2021;143:1642–1654. doi: 10.1161/CIRCULATIONAHA.120.048996. - DOI - PMC - PubMed
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C.N., Mbanya J.C., et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

