Inactivation Effect of Germination Combined with Cold Plasma Treatment on Bacillus licheniformis Spores
- PMID: 38231775
- PMCID: PMC10706397
- DOI: 10.3390/foods12234319
Inactivation Effect of Germination Combined with Cold Plasma Treatment on Bacillus licheniformis Spores
Abstract
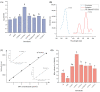
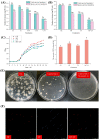
Food spoilage, primarily caused by spore-forming bacteria, has become a critical concern since it results in substantial economic losses within the food industry. Past investigations have successfully identified Bacillus licheniformis as the main bacterium responsible for spoilage in roast chicken. In this study, we screened a new sterilization combination from 16 germinants and 4 cold plasma conditions, respectively. Among them, the combination of "A"GFNa-1 (composed of 60 mmol/L L-alanine, 10 mmol/L D-glucose, 10 mmol/L D-fructose, and 1 g/L NaCl) with cold plasma treatment (packed with 100% argon at 70 kV) proved effective in deactivating B. licheniformis spores, resulting in a reduction of approximately 2.1 log CFU/mL. Furthermore, we exposed the spores to different conditions: CK (no germination, no cold plasma), MF (germination only), CP (no germination, 100% argon packed, 70 kV cold plasma treatment for 3 min), and MF + CP (germination for 5 h, 100% argon packed, 70 kV cold plasma treatment for 3 min). The results of heat inactivation and dipicolinic acid (DPA) release rate demonstrated that cold plasma treatment effectively inactivated both spores and vegetative cells without inducing germination. Additionally, the reduced survival under hyperosmotic conditions and the presence of distinct red fluorescence patterns observed through confocal laser scanning microscopy (CLSM) collectively suggest that cold plasma treatment disrupts the inner membrane structure and leads to the inactivation of B. licheniformis. Overall, our findings indicate a spore clearance rate of 99.2% and suggest that the combination of efficient germinants and cold plasma treatment holds promise as a viable approach to mitigate spore contamination in the food industry.
Keywords: Bacillus licheniformis; cold plasma; germination; inactivation; spores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Synergistic inactivation and mechanism of thermosonication treatment combined with germinants and licorice extracts against Paraclostridium bifermentans.Food Res Int. 2025 Feb;202:115751. doi: 10.1016/j.foodres.2025.115751. Epub 2025 Jan 13. Food Res Int. 2025. PMID: 39967068
-
Effects of High Pressure on Bacillus licheniformis Spore Germination and Inactivation.Appl Environ Microbiol. 2017 Jun 30;83(14):e00503-17. doi: 10.1128/AEM.00503-17. Print 2017 Jul 15. Appl Environ Microbiol. 2017. PMID: 28476768 Free PMC article.
-
The Cooperative and Interdependent Roles of GerA, GerK, and Ynd in Germination of Bacillus licheniformis Spores.Appl Environ Microbiol. 2016 Jun 30;82(14):4279-4287. doi: 10.1128/AEM.00594-16. Print 2016 Jul 15. Appl Environ Microbiol. 2016. PMID: 27208128 Free PMC article.
-
The underlying mechanism of bacterial spore germination: An update review.Compr Rev Food Sci Food Saf. 2023 Jul;22(4):2728-2746. doi: 10.1111/1541-4337.13160. Epub 2023 May 1. Compr Rev Food Sci Food Saf. 2023. PMID: 37125461 Review.
-
Germination of Spores of the Orders Bacillales and Clostridiales.Annu Rev Microbiol. 2017 Sep 8;71:459-477. doi: 10.1146/annurev-micro-090816-093558. Epub 2017 Jul 11. Annu Rev Microbiol. 2017. PMID: 28697670 Review.
Cited by
-
Cold Plasma-Mediated Inactivation of Spore-Forming Microorganisms: Mechanisms, Quality Attributes, and Efficiency Parameters.Food Sci Nutr. 2025 Jun 18;13(6):e70429. doi: 10.1002/fsn3.70429. eCollection 2025 Jun. Food Sci Nutr. 2025. PMID: 40535921 Free PMC article. Review.
References
-
- Petrova L. Diferentsirane na kulturi ot rod Bacillus, izolirani ot mesni polukonservi. Vet. Med. Nauk. 1975;12:82–88. - PubMed
-
- Iacona V.A., Simonetta A.C., Renzulli P.M. Bacteria of genus Bacillus in chicken carcasses and hamburgers. Rev. Argent. Microbiol. 1995;27:21–27. - PubMed
-
- Lopes C., Barbosa J., Maciel E., da Costa E., Alves E., Domingues P., Mendo S., Domingues M.R.M. Lipidomic Signature of Bacillus licheniformis 189 during the Different Growth Phases Unravelled by High-Resolution Liquid Chromatography-Mass Spectrometry. Arch. Biochem. Biophys. 2019;663:83–94. doi: 10.1016/j.abb.2018.12.024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

