Multi-Task Learning for Motion Analysis and Segmentation in 3D Echocardiography
- PMID: 38231820
- PMCID: PMC11514714
- DOI: 10.1109/TMI.2024.3355383
Multi-Task Learning for Motion Analysis and Segmentation in 3D Echocardiography
Abstract
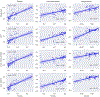
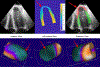
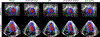
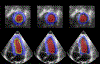
Characterizing left ventricular deformation and strain using 3D+time echocardiography provides useful insights into cardiac function and can be used to detect and localize myocardial injury. To achieve this, it is imperative to obtain accurate motion estimates of the left ventricle. In many strain analysis pipelines, this step is often accompanied by a separate segmentation step; however, recent works have shown both tasks to be highly related and can be complementary when optimized jointly. In this work, we present a multi-task learning network that can simultaneously segment the left ventricle and track its motion between multiple time frames. Two task-specific networks are trained using a composite loss function. Cross-stitch units combine the activations of these networks by learning shared representations between the tasks at different levels. We also propose a novel shape-consistency unit that encourages motion propagated segmentations to match directly predicted segmentations. Using a combined synthetic and in-vivo 3D echocardiography dataset, we demonstrate that our proposed model can achieve excellent estimates of left ventricular motion displacement and myocardial segmentation. Additionally, we observe strong correlation of our image-based strain measurements with crystal-based strain measurements as well as good correspondence with SPECT perfusion mappings. Finally, we demonstrate the clinical utility of the segmentation masks in estimating ejection fraction and sphericity indices that correspond well with benchmark measurements.
Figures




References
-
- Tsao CW et al., “Heart disease and stroke statistics—2022 update: A report from the american heart association,” Circulation, vol. 145, pp. e153–e639, 2022. - PubMed
-
- Reisner S, Lysyansky P, Agmon Y, Mutlak D, Lessick J, and Friedman Z, “Global longitudinal strain: A novel index of left ventricular systolic function,” Journal of the American Society of Echocardiography, vol. 17, pp. 630–3, 06 2004. - PubMed
-
- Papademetris X, Sinusas A, Dione D, Constable R, and Duncan J, “Estimation of 3d left ventricular deformation from medical images using biomechanical models,” IEEE transactions on medical imaging, vol. 21, pp. 786–800, 08 2002. - PubMed
-
- Lin N. and Duncan JS, “Generalized robust point matching using an extended free-form deformation model: application to cardiac images,” in IEEE 2nd International Symposium on Biomedical Imaging, 2004, pp. 320–323 Vol. 1.
-
- Zhu W. et al.., “Neurreg: Neural registration and its application to image segmentation,” IEEE Winter Conference on Applications of Computer Vision, pp. 3606–3615, 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

