On the Mechanism of the Ionizing Radiation-Induced Degradation and Recycling of Cellulose
- PMID: 38231912
- PMCID: PMC10708459
- DOI: 10.3390/polym15234483
On the Mechanism of the Ionizing Radiation-Induced Degradation and Recycling of Cellulose
Abstract
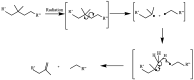
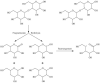
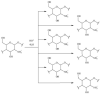
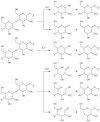
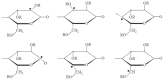
The use of ionizing radiation offers a boundless range of applications for polymer scientists, from inducing crosslinking and/or degradation to grafting a wide variety of monomers onto polymeric chains. This review in particular aims to introduce the field of ionizing radiation as it relates to the degradation and recycling of cellulose and its derivatives. The review discusses the main mechanisms of the radiolytic sessions of the cellulose molecules in the presence and absence of water. During the radiolysis of cellulose, in the absence of water, the primary and secondary electrons from the electron beam, and the photoelectric, Compton effect electrons from gamma radiolysis attack the glycosidic bonds (C-O-C) on the backbone of the cellulose chains. This radiation-induced session results in the formation of alkoxyl radicals and C-centered radicals. In the presence of water, the radiolytically produced hydroxyl radicals (●OH) will abstract hydrogen atoms, leading to the formation of C-centered radicals, which undergo various reactions leading to the backbone session of the cellulose. Based on the structures of the radiolytically produced free radicals in presence and absence of water, covalent grafting of vinyl monomers on the cellulose backbone is inconceivable.
Keywords: cellulose ionizing-radiation; degradation; recycling; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
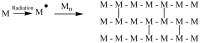




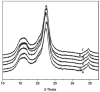
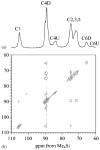
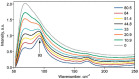
References
-
- Peng B.L., Dhar N., Liu H.L., Tam K.C. Chemistry and Applications of Nanocrystalline Cellulose and Its Derivatives: A Nanotechnology Perspective. Can. J. Chem. Eng. 2011;89:1191–1206. doi: 10.1002/cjce.20554. - DOI
-
- Keshk S., Sameshima K. Influence of Lignosulfonate on Crystal Structure and Productivity of Bacterial Cellulose in a Static Culture. Enzym. Microb. Technol. 2006;40:4–8. doi: 10.1016/j.enzmictec.2006.07.037. - DOI
-
- Brown R.M. The Biosynthesis of Cellulose. J. Macromol. Sci. Part A. 1996;33:1345–1373. doi: 10.1080/10601329608014912. - DOI
Publication types
LinkOut - more resources
Full Text Sources

