Dextran-Based Injectable Hydrogel Composites for Bone Regeneration
- PMID: 38231931
- PMCID: PMC10707775
- DOI: 10.3390/polym15234501
Dextran-Based Injectable Hydrogel Composites for Bone Regeneration
Abstract
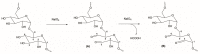
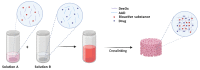
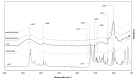
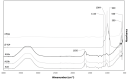
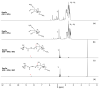
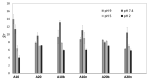
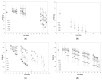
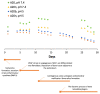
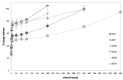
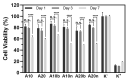
Currently, bone infections caused by diseases or injuries are a major health issue. In addition, the conventional therapeutic approaches used to treat bone diseases or injuries present several drawbacks. In the area of tissue engineering, researchers have been developing new alternative therapeutic approaches, such as scaffolds, to promote the regeneration of injured tissues. Despite the advantages of these materials, most of them require an invasive surgical procedure. To overcome these problems, the main focus of this work was to develop scaffolds for bone regeneration, which can be applied using injectable hydrogels that circumvent the use of invasive procedures, while allowing for bone regeneration. Throughout this work, injectable hydrogels were developed based on a natural polymer, dextran, along with the use of two inorganic compounds, calcium β-triphosphate and nanohydroxyapatite, that aimed to reinforce the mechanical properties of the 3D mesh. The materials were chemically characterized considering the requirements for the intended application: the swelling capacity was evaluated, the degradation rate in a simulated physiological environment was assessed, and compression tests were performed. Furthermore, vancomycin was incorporated into the polymeric matrices to obtain scaffolds with antibacterial performance, and their drug release profile was assessed. The cytotoxic profile of the hydrogels was assessed by an MTS assay, using osteoblasts as model cells. The data obtained demonstrated that dextran-based hydrogels were successfully synthesized, with a drug release profile with an initial burst between 50 and 80% of the drug. The hydrogels possess fair biocompatibility. The swelling capacity showed that the stability of the samples and their degradation profile is compatible with the average time period required for bone regeneration (usually about one month) and have a favorable Young's modulus (200-300 kPa). The obtained hydrogels are well-suited for bone regeneration applications such as infections that occur during implantation or bone graft substitutes with antibiotics.
Keywords: bone regeneration; drug release; injectable hydrogels; oxidized dextran.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

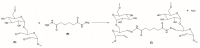
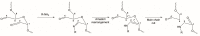




References
Grants and funding
LinkOut - more resources
Full Text Sources

