Kaposi's sarcoma herpesvirus latency-associated nuclear antigen broadly regulates viral gene expression and is essential for lytic infection
- PMID: 38232124
- PMCID: PMC10793894
- DOI: 10.1371/journal.ppat.1011907
Kaposi's sarcoma herpesvirus latency-associated nuclear antigen broadly regulates viral gene expression and is essential for lytic infection
Abstract
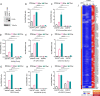
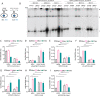
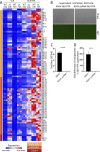
Kaposi's sarcoma herpesvirus (KSHV) is a leading cause of malignancy in AIDS and current therapies are limited. Like all herpesviruses, KSHV infection can be latent or lytic. KSHV latency-associated nuclear antigen (LANA) is essential for viral genome persistence during latent infection. LANA also maintains latency by antagonizing expression and function of the KSHV lytic switch protein, RTA. Here, we find LANA null KSHV is not capable of lytic replication, indicating a requirement for LANA. While LANA promoted both lytic and latent gene expression in cells partially permissive for lytic infection, it repressed expression in non-permissive cells. Importantly, forced RTA expression in non-permissive cells led to induction of lytic infection and LANA switched to promote, rather than repress, most lytic viral gene expression. When basal viral gene expression levels were high, LANA promoted expression, but repressed expression at low basal levels unless RTA expression was forcibly induced. LANA's effects were broad, but virus gene specific, extending to an engineered, recombinant viral GFP under control of host EF1α promoter, but not to host EF1α. Together, these results demonstrate that, in addition to its essential role in genome maintenance, LANA broadly regulates viral gene expression, and is required for high levels of lytic gene expression during lytic infection. Strategies that target LANA are expected to abolish KSHV infection.
Copyright: © 2024 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that they have no competing interests.
Figures




References
-
- Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection [see comments]. The New England journal of medicine. 1995;332(18):1181–5. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi’s Sarcoma-Associated Herpesvirus-Like DNA Sequences in Multicentric Castleman’s Disease. Blood. 1995;86(4):1276–80. - PubMed
-
- Decker LL, Shankar P, Khan G, Freeman RB, Dezube BJ, Lieberman J, et al. The Kaposi sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med. 1996;184(1):283–8. doi: 10.1084/jem.184.1.283 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

