Tissue Organoid Cultures Metabolize Dietary Carcinogens Proficiently and Are Effective Models for DNA Adduct Formation
- PMID: 38232180
- PMCID: PMC10880098
- DOI: 10.1021/acs.chemrestox.3c00255
Tissue Organoid Cultures Metabolize Dietary Carcinogens Proficiently and Are Effective Models for DNA Adduct Formation
Abstract
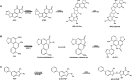
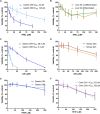
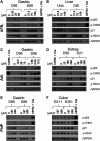
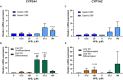
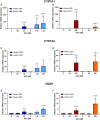
Human tissue three-dimensional (3D) organoid cultures have the potential to reproduce in vitro the physiological properties and cellular architecture of the organs from which they are derived. The ability of organoid cultures derived from human stomach, liver, kidney, and colon to metabolically activate three dietary carcinogens, aflatoxin B1 (AFB1), aristolochic acid I (AAI), and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), was investigated. In each case, the response of a target tissue (liver for AFB1; kidney for AAI; colon for PhIP) was compared with that of a nontarget tissue (gastric). After treatment cell viabilities were measured, DNA damage response (DDR) was determined by Western blotting for p-p53, p21, p-CHK2, and γ-H2AX, and DNA adduct formation was quantified by mass spectrometry. Induction of the key xenobiotic-metabolizing enzymes (XMEs) CYP1A1, CYP1A2, CYP3A4, and NQO1 was assessed by qRT-PCR. We found that organoids from different tissues can activate AAI, AFB1, and PhIP. In some cases, this metabolic potential varied between tissues and between different cultures of the same tissue. Similarly, variations in the levels of expression of XMEs were observed. At comparable levels of cytotoxicity, organoids derived from tissues that are considered targets for these carcinogens had higher levels of adduct formation than a nontarget tissue.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Huch M.; Gehart H.; van Boxtel R.; Hamer K.; Blokzijl F.; Verstegen M. M. A.; Ellis E.; van Wenum M.; Fuchs S. A.; de Ligt J.; van de Wetering M.; Sasaki N.; Boers S. J.; Kemperman H.; de Jonge J.; Ijzermans J. N. M.; Nieuwenhuis E. E. S.; Hoekstra R.; Strom S.; Vries R. R. G.; van der Laan L. J. W.; Cuppen E.; Clevers H. Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell 2015, 160 (1–2), 299–312. 10.1016/j.cell.2014.11.050. - DOI - PMC - PubMed
-
- Garcia A. L. C.; Kucab J. E.; Al-serori H.; Beck R. S. S.; Fischer F.; Hufnagel M.; Hartwig A.; Floeder A.; Balbo S.; Francies H.; Garnett M.; Huch M.; Drost J.; Zilbauer M.; Arlt V. M.; Phillips D. H. Metabolic Activation of Benzo[a]Pyrene by Human Tissue Organoid Cultures. Int. J. Mol. Sci. 2023, 24, 60610.3390/ijms24010606. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

