Quantifying local stiffness and forces in soft biological tissues using droplet optical microcavities
- PMID: 38232279
- PMCID: PMC10823245
- DOI: 10.1073/pnas.2314884121
Quantifying local stiffness and forces in soft biological tissues using droplet optical microcavities
Abstract
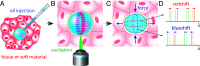
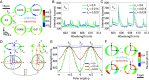
Mechanical properties of biological tissues fundamentally underlie various biological processes and noncontact, local, and microscopic methods can provide fundamental insights. Here, we present an approach for quantifying the local mechanical properties of biological materials at the microscale, based on measuring the spectral shifts of the optical resonances in droplet microcavities. Specifically, the developed method allows for measurements of deformations in dye-doped oil droplets embedded in soft materials or biological tissues with an error of only 1 nm, which in turn enables measurements of anisotropic stress inside tissues as small as a few pN/μm2. Furthermore, by applying an external strain, Young's modulus can be measured in the range from 1 Pa to 35 kPa, which covers most human soft tissues. Using multiple droplet microcavities, our approach could enable mapping of stiffness and forces in inhomogeneous soft tissues and could also be applied to in vivo and single-cell experiments. The developed method can potentially lead to insights into the mechanics of biological tissues.
Keywords: biological tissues; liquid inclusions; microdroplets; stiffness; whispering gallery modes.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
Photo-elasticity of silk fibroin harnessing whispering gallery modes.Sci Rep. 2023 Jun 16;13(1):9750. doi: 10.1038/s41598-023-36400-0. Sci Rep. 2023. PMID: 37328482 Free PMC article.
-
How soft is a single protein? The stress-strain curve of antibody pentamers with 5 pN and 50 pm resolutions.Nanoscale. 2016 Apr 28;8(17):9151-8. doi: 10.1039/c5nr07957h. Nanoscale. 2016. PMID: 26732032
-
Evaluation of an Inverse Method for Quantifying Spatially Variable Mechanics.J Biomech Eng. 2024 Dec 1;146(12):121006. doi: 10.1115/1.4066434. J Biomech Eng. 2024. PMID: 39240274
-
Liquid Droplet Microresonators.Sensors (Basel). 2019 Jan 24;19(3):473. doi: 10.3390/s19030473. Sensors (Basel). 2019. PMID: 30682798 Free PMC article. Review.
-
Method for quantitative measurements of the elastic modulus of biological cells in AFM indentation experiments.Methods. 2013 Apr 1;60(2):202-13. doi: 10.1016/j.ymeth.2013.03.037. Epub 2013 Apr 30. Methods. 2013. PMID: 23639869 Review.
Cited by
-
DNA Sensing with Whispering Gallery Mode Microlasers.Nano Lett. 2025 Mar 19;25(11):4467-4475. doi: 10.1021/acs.nanolett.5c00078. Epub 2025 Mar 4. Nano Lett. 2025. PMID: 40035381 Free PMC article.
References
-
- P. G. De Gennes, J. Prost, The Physics of Liquid Crystals (Oxford University Press, 1993), vol. 83.
-
- Kimura Y., Microrheology of soft matter. J. Phys. Soc. Jpn. 78, 041005 (2009).
-
- Levental I., Georges P. C., Janmey P. A., Soft biological materials and their impact on cell function. Soft Matter 3, 299–306 (2007). - PubMed
-
- Cheung H. K., et al. , Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 35, 1914–1923 (2014). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

