The hepatokine FGL1 regulates hepcidin and iron metabolism during anemia in mice by antagonizing BMP signaling
- PMID: 38232308
- PMCID: PMC11103088
- DOI: 10.1182/blood.2023022724
The hepatokine FGL1 regulates hepcidin and iron metabolism during anemia in mice by antagonizing BMP signaling
Abstract
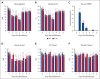
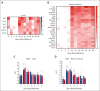
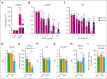
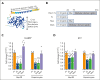
As a functional component of erythrocyte hemoglobin, iron is essential for oxygen delivery to all tissues in the body. The liver-derived peptide hepcidin is the master regulator of iron homeostasis. During anemia, the erythroid hormone erythroferrone regulates hepcidin synthesis to ensure the adequate supply of iron to the bone marrow for red blood cell production. However, mounting evidence suggested that another factor may exert a similar function. We identified the hepatokine fibrinogen-like 1 (FGL1) as a previously undescribed suppressor of hepcidin that is induced in the liver in response to hypoxia during the recovery from anemia, and in thalassemic mice. We demonstrated that FGL1 is a potent suppressor of hepcidin in vitro and in vivo. Deletion of Fgl1 in mice results in higher hepcidin levels at baseline and after bleeding. FGL1 exerts its activity by directly binding to bone morphogenetic protein 6 (BMP6), thereby inhibiting the canonical BMP-SMAD signaling cascade that controls hepcidin transcription.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: T.G. and E.N. are scientific cofounders of Intrinsic LifeSciences and Silarus Therapeutics. T.G. is a consultant for ADARx, Akebia, Pharmacosmos, Ionis, Gossamer Bio, Global Blood Therapeutics, American Regent, Disc Medicine, RallyBio, and Rockwell Scientific. E.N. is a consultant for Protagonist, Vifor, RallyBio, Ionis, GlaxoSmithKline, Novo Nordisk, AstraZeneca FibroGen, and Disc Medicine. Y.Z.G. is a consultant for Ionis, Protagonist, Denali/Takeda, and Bay Clinical. The remaining authors declare no competing financial interests.
Figures



Update of
-
The hepatokine FGL1 regulates hepcidin and iron metabolism during the recovery from hemorrhage-induced anemia in mice.bioRxiv [Preprint]. 2023 Apr 6:2023.04.06.535920. doi: 10.1101/2023.04.06.535920. bioRxiv. 2023. Update in: Blood. 2024 Mar 28;143(13):1282-1292. doi: 10.1182/blood.2023022724. PMID: 37066218 Free PMC article. Updated. Preprint.
Comment in
-
Ironing erythroid cells takes FLG1 and ERFE to tango.Blood. 2024 Mar 28;143(13):1208-1209. doi: 10.1182/blood.2023023645. Blood. 2024. PMID: 38546639 No abstract available.
References
-
- Camaschella C. Iron deficiency. Blood. 2019;133(1):30–39. - PubMed
-
- Finch C. Regulators of iron balance in humans. Blood. 1994;84(6):1697–1702. - PubMed
-
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

