Migraine-Related Stigma and Its Relationship to Disability, Interictal Burden, and Quality of Life: Results of the OVERCOME (US) Study
- PMID: 38232340
- PMCID: PMC11097761
- DOI: 10.1212/WNL.0000000000208074
Migraine-Related Stigma and Its Relationship to Disability, Interictal Burden, and Quality of Life: Results of the OVERCOME (US) Study
Abstract
Background and objectives: This population-based analysis characterizes the relative frequency of migraine-related stigma and its cross-sectional relationship to migraine outcomes. We hypothesized that migraine-related stigma would be inversely associated with favorable migraine outcomes across headache day categories.
Methods: OVERCOME (US) is a web-based observational study that annually recruited a demographically representative US sample and then identified people with active migraine using a validated migraine diagnostic questionnaire. It also assessed how frequently respondents experienced migraine-related stigma using a novel 12-item questionnaire (Migraine-Related Stigma, MiRS) that contained 2 factors; feeling that others viewed migraine as being used for Secondary Gain (8 items, α = 0.92) and feeling that others were Minimizing disease Burden (4 items, α = 0.86). We defined 5 groups: (1) MiRS-Both (Secondary Gain and Minimizing Burden often/very often; (2) MiRS-SG (Secondary Gain often/very often); (3) MiRS-MB (Minimizing Burden often/very often); (4) MiRS-Rarely/Sometimes; (5) MiRS-Never. Using MiRS group as the independent variable, we modeled its cross-sectional relationship to disability (Migraine Disability Assessment, MIDAS), interictal burden (Migraine Interictal Burden Scale-4), and migraine-specific quality of life (Migraine Specific Quality of Life v2.1 Role Function-Restrictive) while controlling for sociodemographics, clinical features, and monthly headache day categories.
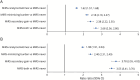
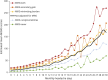
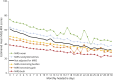
Results: Among this population-based sample with active migraine (n = 59,001), mean age was 41.3 years and respondents predominantly identified as female (74.9%) and as White (70.1%). Among respondents, 41.1% reported experiencing, on average, ≥4 monthly headache days and 31.7% experienced migraine-related stigma often/very often; the proportion experiencing migraine-related stigma often/very often increased from 25.5% among those with <4 monthly headache days to 47.5% among those with ≥15 monthly headache days. The risk for increased disability (MIDAS score) was significant for each MiRS group compared with the MiRS-Never group; the risk more than doubled for the MiRS-Both group (rate ratio 2.68, 95% CI 2.56-2.80). For disability, interictal burden, and migraine-specific quality of life, increased migraine-related stigma was associated with increased disease burden across all monthly headache day categories.
Discussion: OVERCOME (US) found that 31.7% of people with migraine experienced migraine-related stigma often/very often and was associated with more disability, greater interictal burden, and reduced quality of life.
Conflict of interest statement
R.E. Shapiro serves as consultant, advisory board member, or has received honoraria or research support from Eli Lilly and Company, Lundbeck, and Theranica. R.A. Nicholson is an employee and minor stockholder of Eli Lilly and Company. E.K. Seng has consulted or served on an advisory board for GlaxoSmithKline, Click Therapeutics, and Abbvie and received research funding from the National Institute of Neurological Disorders and Stroke (NS096107 PI: Seng), NCCIH (R01AT011005-01A1 MPIs: Seng and Shallcross), and the Veteran's Health Administration (the Headache Center of Excellence Research and Evaluation Center and VA HSR&D, IRP 20-002 PI: Damush). D.C. Buse has received research support from the FDA and the National Headache Foundation. She serves as consultant, advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, Collegium, Eli Lilly and Company, Lundbeck, Novartis, and Teva. M.L. Reed has received research support from the National Headache Foundation. He serves as consultant, advisory board member, or has received honoraria or research support from Abbvie/Allergan, Amgen, Dr. Reddy's Laboratories (Promius), and Eli Lilly and Company. A.J. Zagar, MS, is an employee and minor stockholder of Eli Lilly and Company. S. Ashina consulting, teaching, honoraria: Allergan, Amgen, Biohaven Pharmaceuticals, Eli Lilly and Company, Impel NeuroPharma, Novartis, Satsuma, Supernus, Percept, and Theranica. E.J. Muenzel is an employee and minor stockholder of Eli Lilly and Company. S. Hutchinson consulting, speaking, honoraria from Alder/Lundbeck, AbbVie/Allergan, Amgen, Biohaven, Currax, electroCore, Eli Lilly and Company, Impel, Novartis, Teva, Theranica, and Upsher-Smith. E.M. Pearlman is an employee and minor stockholder of Eli Lilly and Company. R.B. Lipton has received research support from the NIH, the FDA, and the National Headache Foundation. He serves as consultant, advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, Dr. Reddy's Laboratories (Promius), electroCore, Eli Lilly and Company, GlaxoSmithKline, Lundbeck, Manistee, Novartis, Teva, Vector, and Vedanta Research. He receives royalties from Wolff's Headache, 8th edition (Oxford University Press, 2009) and Informa. He holds stock/options in Biohaven and Manistee. Go to
Figures


References
-
- Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z; Lifting The Burden: the Global Campaign against Headache. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. doi:10.1186/s10194-020-01208-0 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
