Thioredoxin (Trx): A redox target and modulator of cellular senescence and aging-related diseases
- PMID: 38232457
- PMCID: PMC10827563
- DOI: 10.1016/j.redox.2024.103032
Thioredoxin (Trx): A redox target and modulator of cellular senescence and aging-related diseases
Abstract
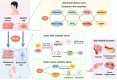
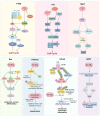
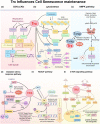
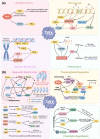
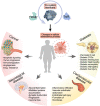
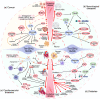
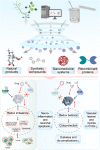
Thioredoxin (Trx) is a compact redox-regulatory protein that modulates cellular redox state by reducing oxidized proteins. Trx exhibits dual functionality as an antioxidant and a cofactor for diverse enzymes and transcription factors, thereby exerting influence over their activity and function. Trx has emerged as a pivotal biomarker for various diseases, particularly those associated with oxidative stress, inflammation, and aging. Recent clinical investigations have underscored the significance of Trx in disease diagnosis, treatment, and mechanistic elucidation. Despite its paramount importance, the intricate interplay between Trx and cellular senescence-a condition characterized by irreversible growth arrest induced by multiple aging stimuli-remains inadequately understood. In this review, our objective is to present a comprehensive and up-to-date overview of the structure and function of Trx, its involvement in redox signaling pathways and cellular senescence, its association with aging and age-related diseases, as well as its potential as a therapeutic target. Our review aims to elucidate the novel and extensive role of Trx in senescence while highlighting its implications for aging and age-related diseases.
Keywords: Age-related diseases; Anti-oxidative; Cellular senescence; ROS; Thioredoxin.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Kovatcheva M., Liao W., Klein M.E., Robine N., Geiger H., Crago A.M., et al. ATRX is a regulator of therapy induced senescence in human cells. Nat. Commun. 2017;8(1):386. doi: 10.1038/s41467-017-00540-5. https://www.ncbi.nlm.nih.gov/pubmed/28855512 - DOI - PMC - PubMed
-
- Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 2015;21(12):1424–1435. doi: 10.1038/nm.4000. https://www.ncbi.nlm.nih.gov/pubmed/26646499 - DOI - PMC - PubMed
-
- Holmstrom K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. https://www.ncbi.nlm.nih.gov/pubmed/24854789 - DOI - PubMed
-
- Nobrega-Pereira S., Fernandez-Marcos P.J., Brioche T., Gomez-Cabrera M.C., Salvador-Pascual A., Flores J.M., et al. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016;7:10894. doi: 10.1038/ncomms10894. https://www.ncbi.nlm.nih.gov/pubmed/26976705 - DOI - PMC - PubMed
-
- Lee J.H., Jung M., Hong J., Kim M.K., Chung I.K. Loss of RNA-binding protein HuR facilitates cellular senescence through posttranscriptional regulation of TIN2 mRNA. Nucleic Acids Res. 2018;46(8):4271–4285. doi: 10.1093/nar/gky223. https://www.ncbi.nlm.nih.gov/pubmed/29584879 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

