The characteristics of pre-existing humoral imprint determine efficacy of S. aureus vaccines and support alternative vaccine approaches
- PMID: 38232694
- PMCID: PMC10829788
- DOI: 10.1016/j.xcrm.2023.101360
The characteristics of pre-existing humoral imprint determine efficacy of S. aureus vaccines and support alternative vaccine approaches
Abstract
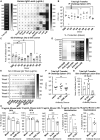
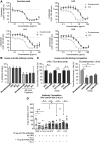
The failure of the Staphylococcus aureus (SA) IsdB vaccine trial can be explained by the recall of non-protective immune imprints from prior SA exposure. Here, we investigate natural human SA humoral imprints to understand their broader impact on SA immunizations. We show that antibody responses against SA cell-wall-associated antigens (CWAs) are non-opsonic, while antibodies against SA toxins are neutralizing. Importantly, the protective characteristics of the antibody imprints accurately predict the failure of corresponding vaccines against CWAs and support vaccination against toxins. In passive immunization platforms, natural anti-SA human antibodies reduce the efficacy of the human monoclonal antibodies suvratoxumab and tefibazumab, consistent with the results of their respective clinical trials. Strikingly, in the absence of specific humoral memory responses, active immunizations are efficacious in both naive and SA-experienced mice. Overall, our study points to a practical and predictive approach to evaluate and develop SA vaccines based on pre-existing humoral imprint characteristics.
Keywords: S. aureus; antibody interference; immune imprinting; immunization; original antigenic sin; vaccine.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.Y., C.-M.T., and I.A.H. have received a research grant from Pfizer to study SA vaccines.
Figures




References
-
- CDC . 2018. Biggest Threats and Data.https://www.cdc.gov/drugresistance/biggest_threats.html?CDC_AA_refVal=ht...

