Lipoprotein(a) Is Markedly More Atherogenic Than LDL: An Apolipoprotein B-Based Genetic Analysis
- PMID: 38233012
- PMCID: PMC7616706
- DOI: 10.1016/j.jacc.2023.10.039
Lipoprotein(a) Is Markedly More Atherogenic Than LDL: An Apolipoprotein B-Based Genetic Analysis
Abstract
Background: Lipoprotein(a) (Lp(a)) is recognized as a causal factor for coronary heart disease (CHD) but its atherogenicity relative to that of low-density lipoprotein (LDL) on a per-particle basis is indeterminate.
Objectives: The authors addressed this issue in a genetic analysis based on the fact that Lp(a) and LDL both contain 1 apolipoprotein B (apoB) per particle.
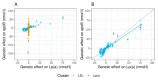
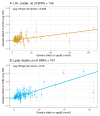
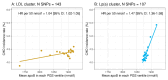
Methods: Genome-wide association studies using the UK Biobank population identified 2 clusters of single nucleotide polymorphisms: one comprising 107 variants linked to Lp(a) mass concentration, the other with 143 variants linked to LDL concentration. In these Lp(a) and LDL clusters, the relationship of genetically predicted variation in apoB with CHD risk was assessed.
Results: The Mendelian randomization-derived OR for CHD for a 50 nmol/L higher Lp(a)-apoB was 1.28 (95% CI: 1.24-1.33) compared with 1.04 (95% CI: 1.03-1.05) for the same increment in LDL-apoB. Likewise, use of polygenic scores to rank subjects according to difference in Lp(a)-apoB vs difference in LDL-apoB revealed a greater HR for CHD per 50 nmol/L apoB for the Lp(a) cluster (1.47; 95% CI: 1.36-1.58) compared with the LDL cluster (1.04; 95% CI: 1.02-1.05). From these data, we estimate that the atherogenicity of Lp(a) is approximately 6-fold (point estimate of 6.6; 95% CI: 5.1-8.8) greater than that of LDL on a per-particle basis.
Conclusions: We conclude that the atherogenicity of Lp(a) (CHD risk quotient per unit increase in particle number) is substantially greater than that of LDL. Therefore, Lp(a) represents a key target for drug-based intervention in a significant proportion of the at-risk population.
Keywords: LDL cholesterol; Mendelian randomization; UK Biobank; cardiovascular disease; lipoprotein(a).
Copyright © 2024 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures The work in this paper was supported by grants from Swedish Research Council, Swedish Heart Lung Foundation, from the Swedish state under the agreement between the Swedish government and the county councils; the ALF-agreement (ALFGBG-965404), Sigrid Juselius Foundation, Helsinki, Finland, and the Finnish Foundation for Cardiovascular Research. Dr Burgess is supported by the Wellcome Trust (225790/Z/22/Z). This research was funded by United Kingdom Research and Innovation Medical Research Council (MC_UU_00002/7) and supported by the National Institute for Health Research Cambridge Biomedical Research Centre (BRC-1215-20014). This research has been conducted using the open-access UK Biobank Resource under application number [53308]. Thus, the study is exempt from ethical review board approval. Dr Taskinen has received honoraria from Novartis, Akcea, Amgen, Novo Nordisk, Mylan, Chiesi Pharma, and Eli Lilly. Dr Borén has received honoraria from Novartis, Novo Nordisk, Akcea, Amgen, and Pfizer. Dr Chapman has received honoraria from Amgen, Amarin, AstraZeneca, Daiichi-Sankyo, Kowa, Lexicon, MSD, Regeneron, Sanofi, and Pfizer. Dr Packard has received honoraria from Amgen, Amarin, MSD, Dalcor, Novartis, and Daiichi-Sankyo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Comment in
-
Particle Number and Characteristics of Lipoprotein(a), LDL, and apoB: Perspectives on Contributions to ASCVD.J Am Coll Cardiol. 2024 Jan 23;83(3):396-400. doi: 10.1016/j.jacc.2023.11.008. J Am Coll Cardiol. 2024. PMID: 38233013 No abstract available.
References
-
- Reyes-Soffer G, Ginsberg HN, Berglund L, et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e48–e60. doi: 10.1161/ATV.0000000000000147. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

