Acute rehabilitation following traumatic anterior shoulder dislocation (ARTISAN): pragmatic, multicentre, randomised controlled trial
- PMID: 38233068
- PMCID: PMC10792684
- DOI: 10.1136/bmj-2023-076925
Acute rehabilitation following traumatic anterior shoulder dislocation (ARTISAN): pragmatic, multicentre, randomised controlled trial
Erratum in
-
Acute rehabilitation following traumatic anterior shoulder dislocation (ARTISAN): pragmatic, multicentre, randomised controlled trial.BMJ. 2024 Mar 15;384:q662. doi: 10.1136/bmj.q662. BMJ. 2024. PMID: 38490685 Free PMC article. No abstract available.
Abstract
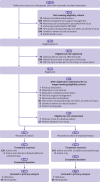
Objective: To assess the effects of an additional programme of physiotherapy in adults with a first-time traumatic shoulder dislocation compared with single session of advice, supporting materials, and option to self-refer to physiotherapy.
Design: Pragmatic, multicentre, randomised controlled trial (ARTISAN).
Setting and participants: Trauma research teams at 41 UK NHS Trust sites screened adults with a first time traumatic anterior shoulder dislocation confirmed radiologically, being managed non-operatively. People were excluded if they presented with both shoulders dislocated, had a neurovascular complication, or were considered for surgical management.
Interventions: One session of advice, supporting materials, and option to self-refer to physiotherapy (n=240) was assessed against the same advice and supporting materials and an additional programme of physiotherapy (n=242). Analyses were on an intention-to-treat basis with secondary per protocol analyses.
Main outcome measures: The primary outcome was the Oxford shoulder instability score (a single composite measure of shoulder function), measured six months after treatment allocation. Secondary outcomes included the QuickDASH, EQ-5D-5L, and complications.
Results: 482 participants were recruited from 40 sites in the UK. 354 (73%) participants completed the primary outcome score (n=180 allocated to advice only, n=174 allocated to advice and physiotherapy). Participants were mostly male (66%), with a mean age of 45 years. No significant difference was noted between advice compared with advice and a programme of physiotherapy at six months for the primary intention-to-treat adjusted analysis (between group difference favouring physiotherapy 1.5 (95% confidence interval -0.3 to 3.5)) or at earlier three month and six week timepoints. Complication profiles were similar across the two groups (P>0.05).
Conclusions: An additional programme of current physiotherapy is not superior to advice, supporting materials, and the option to self-refer to physiotherapy.
Trial registration: Current Controlled Trials ISRCTN63184243.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: all authors had financial support from National Institute for Health Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. RSK is co-chair of the NIHR Programme Grants for Applied Research committee, a paid position in NIHR but unrelated to the trial. She is also a previous chair of the NIHR West Midlands Research for Patient Benefit ( committee and member of the NIHR Health Technology Assessment Clinical Evaluation and Trials Committee and NIHR Integrated Clinical Academic doctoral committee. RSK, DE, HP, AH, JM, HN, SD, CM, HB, DT, and MU have all been awarded current and previous NIHR research grants. HP, MU, and RSK are co-investigators on grants funded by the Australian NHMRC and NIHR funded studies receiving additional support from Stryker Ltd MU is a co-investigator on a grant funded by Norwegian Medical Research Council. He is a director and shareholder of Clinvivo Ltd that provides electronic data collection for health services research. He is part of an academic partnership with Serco Ltd, funded by the European Social Fund, related to return-to-work initiatives. They declare that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- National Institute for Health and Care Excellence. Falls in older people: assessing risk and prevention (CG161). 2013. https://www.nice.org.uk/guidance/cg161 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical