Pragmatic targets for moderate/severe SLE and their implications for clinical care and trial design: sustained DORIS or LLDAS for at least 6 months is sufficient while their attainment for at least 24 months ensures high specificity for damage-free progression
- PMID: 38233103
- PMCID: PMC10958283
- DOI: 10.1136/ard-2023-224919
Pragmatic targets for moderate/severe SLE and their implications for clinical care and trial design: sustained DORIS or LLDAS for at least 6 months is sufficient while their attainment for at least 24 months ensures high specificity for damage-free progression
Abstract
Objectives: Treatment targets in systemic lupus erythematosus (SLE) have been validated in unselected-in terms of severity-cohorts, which limits their generalisability. We assessed remission (Definition of Remission in SLE (DORIS)) and Lupus Low Disease Activity State (LLDAS) in a historical cohort of 348 patients with active moderate-to-severe disease and median follow-up of 5 years.
Methods: Active SLE was defined as Physician Global Assessment ≥1.5 and/or SLE Disease Activity Index 2000 ≥6, requiring therapy intensification. DORIS/LLDAS, organ damage, flares and adverse events were monitored. Shared frailty survival, generalised linear models and K-means clustering were applied.
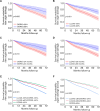
Results: Sustained DORIS and LLDAS for ≥6 months occurred in 41.1% and 80.4%, respectively, and resulted in reduced damage accrual (HR: 0.58; 95% CI 0.36 to 0.93 and 0.61; 0.43 to 0.86) and severe flares (HR: 0.14; 0.08 to 0.27 and 0.19; 0.13 to 0.27). LLDAS without DORIS was also protective (HR: 0.65; 0.43 to 0.98 for damage, 0.49; 0.36 to 0.67 for flares). Models fitting increasing duration of targets showed that DORIS ≥50% and LLDAS ≥60% of time, or alternatively, ≥24 and ≥36 months, achieved optimal balance between feasibility (20.2-41.7%) and specificity (73.3-86.1%) for damage-free outcome. These targets were linked to reduced serious adverse events (risk ratio (RR): 0.56-0.71), hospitalisation (RR: 0.70) and mortality (RR: 0.06-0.13). Patients with predominant arthritis and mucocutaneous disease experienced reduced DORIS/LLDAS, compared with counterparts with major organ involvement. Conventional drugs were more frequently used in the former group, whereas potent immunosuppressive/biological agents in the latter.
Conclusions: In moderate-to-severe SLE, sustained DORIS/LLDAS for at least 6 months is sufficient, while attainment for at least 24 months ensures higher specificity for damage-free progression, thus facilitating treat-to-target strategies and clinical trials. Arthritis and skin disease represent unmet therapeutic needs that could benefit from novel biologics.
Keywords: Lupus Erythematosus, Systemic; Outcome Assessment, Health Care; Therapeutics.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ES received consulting fees from AstraZeneca out of the present work. AF reports honoraria and/or consulting fees from Lilly, Boehringer, Novartis, AbbVie, AstraZeneca, GSK, MSD, Pfizer, UCB, Amgen and Aenorasis, and support for attending meetings from UCB. MG has received fees for sponsored lectures from GSK and AstraZeneca. DTB reports unrestricted investigational grants from GSK, and honoraria and/or consulting fees from GSK, AstraZeneca and Pfizer. AB reports consulting fees from GSK. GB reports grants from GSK, AstraZeneca and Pfizer; honoraria and/or consulting fees from Lilly, Aenorasis, Novartis, AstraZeneca, GSK, SOBI and Pfizer; and participation in advisory boards from Novartis. The remaining authors declare no conflict of interest. One of the coauthors (DTB) is a member of the journal's editorial board.
Figures

Similar articles
-
Evaluation of remission definitions for systemic lupus erythematosus: a prospective cohort study.Lancet Rheumatol. 2019 Oct;1(2):e103-e110. doi: 10.1016/S2665-9913(19)30048-7. Epub 2019 Sep 25. Lancet Rheumatol. 2019. PMID: 38229337
-
Treat-to-target in SLE: is serology important? Results from an integrated analysis of five randomized clinical trials of belimumab.Rheumatology (Oxford). 2025 Jun 1;64(6):3598-3605. doi: 10.1093/rheumatology/keaf107. Rheumatology (Oxford). 2025. PMID: 39985454
-
Targeting DORIS Remission and LLDAS in SLE: A Review.Rheumatol Ther. 2023 Dec;10(6):1459-1477. doi: 10.1007/s40744-023-00601-w. Epub 2023 Oct 5. Rheumatol Ther. 2023. PMID: 37798595 Free PMC article. Review.
-
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials.Ann Rheum Dis. 2025 May;84(5):777-788. doi: 10.1016/j.ard.2025.01.016. Epub 2025 Jan 30. Ann Rheum Dis. 2025. PMID: 39948001 Clinical Trial.
-
Caveats and pitfalls in defining low disease activity in systemic lupus erythematosus.Autoimmun Rev. 2022 Oct;21(10):103165. doi: 10.1016/j.autrev.2022.103165. Epub 2022 Aug 2. Autoimmun Rev. 2022. PMID: 35931316 Review.
Cited by
-
Evolving Concepts in Treat-to-Target Strategies for Systemic Lupus Erythematosus.Mediterr J Rheumatol. 2024 Jun 30;35(Suppl 2):328-341. doi: 10.31138/mjr.290424.eci. eCollection 2024 Jun. Mediterr J Rheumatol. 2024. PMID: 39193182 Free PMC article. Review.
-
Proceedings of the 1st Symposium "Autoimmune Diseases: Clinical Unmet Needs in Systemic Autoimmune Diseases Guide Clinical, Translational and Basic Research".Mediterr J Rheumatol. 2024 Dec 31;35(4):692-703. doi: 10.31138/mjr.121124.pts. eCollection 2024 Dec. Mediterr J Rheumatol. 2024. PMID: 39886294 Free PMC article. No abstract available.
-
Combination of clinical factors predicts successful glucocorticoid withdrawal in systemic lupus erythematosus (SLE): results from a multicentre, retrospective cohort study.RMD Open. 2025 Jan 6;11(1):e005118. doi: 10.1136/rmdopen-2024-005118. RMD Open. 2025. PMID: 39762120 Free PMC article.
-
Outcomes following immunosuppressive therapy withdrawal after complete renal response in proliferative lupus nephritis.Lupus Sci Med. 2025 Jan 19;12(1):e001375. doi: 10.1136/lupus-2024-001375. Lupus Sci Med. 2025. PMID: 39832909 Free PMC article.
-
Glucocorticoids discontinuation in systemic lupus erythematosus: a single-centre study.Rheumatol Adv Pract. 2025 Mar 31;9(2):rkaf036. doi: 10.1093/rap/rkaf036. eCollection 2025. Rheumatol Adv Pract. 2025. PMID: 40342521 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

