Enhanced Photodynamic Therapy Synergizing with Inhibition of Tumor Neutrophil Ferroptosis Boosts Anti-PD-1 Therapy of Gastric Cancer
- PMID: 38233204
- PMCID: PMC10966534
- DOI: 10.1002/advs.202307870
Enhanced Photodynamic Therapy Synergizing with Inhibition of Tumor Neutrophil Ferroptosis Boosts Anti-PD-1 Therapy of Gastric Cancer
Abstract
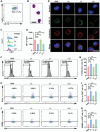
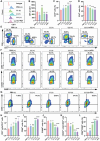
For tumor treatment, the ultimate goal in tumor therapy is to eliminate the primary tumor, manage potential metastases, and trigger an antitumor immune response, resulting in the complete clearance of all malignant cells. Tumor microenvironment (TME) refers to the local biological environment of solid tumors and has increasingly become an attractive target for cancer therapy. Neutrophils within TME of gastric cancer (GC) spontaneously undergo ferroptosis, and this process releases oxidized lipids that limit T cell activity. Enhanced photodynamic therapy (PDT) mediated by di-iodinated IR780 (Icy7) significantly increases the production of reactive oxygen species (ROS). Meanwhile, neutrophil ferroptosis can be triggered by increased ROS generation in the TME. In this study, a liposome encapsulating both ferroptosis inhibitor Liproxstatin-1 and modified photosensitizer Icy7, denoted LLI, significantly inhibits tumor growth of GC. LLI internalizes into MFC cells to generate ROS causing immunogenic cell death (ICD). Simultaneously, liposome-deliver Liproxstatin-1 effectively inhibits the ferroptosis of tumor neutrophils. LLI-based immunogenic PDT and neutrophil-targeting immunotherapy synergistically boost the anti-PD-1 treatment to elicit potent TME and systemic antitumor immune response with abscopal effects. In conclusion, LLI holds great potential for GC immunotherapy.
Keywords: ferroptosis; gastric cancer; immunotherapy; neutrophils; photodynamic therapy.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., CA Cancer J. Clin. 2021, 71, 209. - PubMed
-
- Shitara K., Ajani J. A., Moehler M., Garrido M., Gallardo C., Shen L., Yamaguchi K., Wyrwicz L., Skoczylas T., Bragagnoli A. C., Liu T., Tehfe M., Elimova E., Bruges R., Zander T., De Azevedo S., Kowalyszyn R., Pazo‐Cid R., Schenker M., Cleary J. M., Yanez P., Feeney K., Karamouzis M. V., Poulart V., Lei M., Xiao H., Kondo K., Li M., Janjigian Y. Y., Nature 2022, 603, 942. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2022ZB704/Jiangsu Funding Program for Excellent Postdoctoral Talent
- 82172645/National Natural Science Foundation of China
- 82372805/National Natural Science Foundation of China
- BE2022667/Key Research and Development Program of Jiangsu Province
- BE2022753/Key Research and Development Program of Jiangsu Province
- ZKX21013/Key Project of Nanjing Health Commission
- 2023-JCYJ-YP-02/Nanjing Drum Tower Hospital Cultivation Program for Outstanding Young Science Fund of National Natural Science Foundation of China
- 2021-LCYJ-MS-09/Clinical Trials from the Affiliated Drum Tower Hospital Medical School of Nanjing University
- 2021-LCYJ-PY-17/Clinical Trials from the Affiliated Drum Tower Hospital Medical School of Nanjing University
- BK20200052/Natural Science Foundation of Jiangsu Province for Outstanding Youth Scholars
- 05002/Bethune Charitable Foundation
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
