Clinical chorioamnionitis at term: definition, pathogenesis, microbiology, diagnosis, and treatment
- PMID: 38233317
- PMCID: PMC11288098
- DOI: 10.1016/j.ajog.2023.02.002
Clinical chorioamnionitis at term: definition, pathogenesis, microbiology, diagnosis, and treatment
Abstract
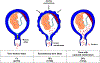
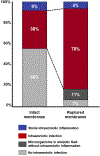
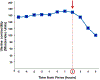
Clinical chorioamnionitis, the most common infection-related diagnosis in labor and delivery units, is an antecedent of puerperal infection and neonatal sepsis. The condition is suspected when intrapartum fever is associated with two other maternal and fetal signs of local or systemic inflammation (eg, maternal tachycardia, uterine tenderness, maternal leukocytosis, malodorous vaginal discharge or amniotic fluid, and fetal tachycardia). Clinical chorioamnionitis is a syndrome caused by intraamniotic infection, sterile intraamniotic inflammation (inflammation without bacteria), or systemic maternal inflammation induced by epidural analgesia. In cases of uncertainty, a definitive diagnosis can be made by analyzing amniotic fluid with methods to detect bacteria (Gram stain, culture, or microbial nucleic acid) and inflammation (white blood cell count, glucose concentration, interleukin-6, interleukin-8, matrix metalloproteinase-8). The most common microorganisms are Ureaplasma species, and polymicrobial infections occur in 70% of cases. The fetal attack rate is low, and the rate of positive neonatal blood cultures ranges between 0.2% and 4%. Intrapartum antibiotic administration is the standard treatment to reduce neonatal sepsis. Treatment with ampicillin and gentamicin have been recommended by professional societies, although other antibiotic regimens, eg, cephalosporins, have been used. Given the importance of Ureaplasma species as a cause of intraamniotic infection, consideration needs to be given to the administration of antimicrobial agents effective against these microorganisms such as azithromycin or clarithromycin. We have used the combination of ceftriaxone, clarithromycin, and metronidazole, which has been shown to eradicate intraamniotic infection with microbiologic studies. Routine testing of neonates born to affected mothers for genital mycoplasmas could improve the detection of neonatal sepsis. Clinical chorioamnionitis is associated with decreased uterine activity, failure to progress in labor, and postpartum hemorrhage; however, clinical chorioamnionitis by itself is not an indication for cesarean delivery. Oxytocin is often administered for labor augmentation, and it is prudent to have uterotonic agents at hand to manage postpartum hemorrhage. Infants born to mothers with clinical chorioamnionitis near term are at risk for early-onset neonatal sepsis and for long-term disability such as cerebral palsy. A frontier is the noninvasive assessment of amniotic fluid to diagnose intraamniotic inflammation with a transcervical amniotic fluid collector and a rapid bedside test for IL-8 for patients with ruptured membranes. This approach promises to improve diagnostic accuracy and to provide a basis for antimicrobial administration.
Keywords: Batson's plexus; Gardnerella vaginalis; NLRP3 inflammasome; Ureaplasma species; abnormal fetal heart rate pattern; adverse maternal outcome; adverse neonatal outcome; amniocentesis; amniotic fluid; ampicillin; antibiotics; antipyretics; azithromycin; biomarker; carbapenem; cerebral palsy; chemokine; clarithromycin; clavulanic acid; cytokine; epidural; fetal heart rate tracing; fetal inflammatory response syndrome; fetal tachycardia; fever; funisitis; genital mycoplasma; gentamycin; histologic chorioamnionitis; infection; inflammasome; interleukin-6 (IL-6); interleukin-8 (IL-8); intraamniotic infection; intraamniotic inflammation; intrapartum fever; maternal N-acetylcysteine (NAC); maternal leukocytosis; matrix metalloproteinase-8 (MMP-8); neonatal bacteremia; neonatal sepsis; overshoot; piperacillin; postpartum hemorrhage; pyrogenic; sterile intraamniotic inflammation; tazobactam, ticarcillin.
Published by Elsevier Inc.
Conflict of interest statement
Figures











References
-
- Alexander JM , McIntire DM , Leveno KJ. Chorioamnionitis and the prognosis for term infants. Obstet Gynecol 1999;94:274–8. - PubMed
-
- Rouse DJ , Landon M , Leveno KJ, et al. The Maternal-Fetal Medicine Units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol 2004;191:211–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

