The mitochondrial ATP-dependent potassium channel (mitoKATP) controls skeletal muscle structure and function
- PMID: 38233399
- PMCID: PMC10794173
- DOI: 10.1038/s41419-024-06426-x
The mitochondrial ATP-dependent potassium channel (mitoKATP) controls skeletal muscle structure and function
Abstract
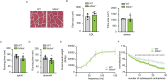
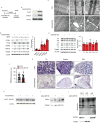
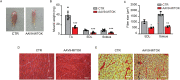
MitoKATP is a channel of the inner mitochondrial membrane that controls mitochondrial K+ influx according to ATP availability. Recently, the genes encoding the pore-forming (MITOK) and the regulatory ATP-sensitive (MITOSUR) subunits of mitoKATP were identified, allowing the genetic manipulation of the channel. Here, we analyzed the role of mitoKATP in determining skeletal muscle structure and activity. Mitok-/- muscles were characterized by mitochondrial cristae remodeling and defective oxidative metabolism, with consequent impairment of exercise performance and altered response to damaging muscle contractions. On the other hand, constitutive mitochondrial K+ influx by MITOK overexpression in the skeletal muscle triggered overt mitochondrial dysfunction and energy default, increased protein polyubiquitination, aberrant autophagy flux, and induction of a stress response program. MITOK overexpressing muscles were therefore severely atrophic. Thus, the proper modulation of mitoKATP activity is required for the maintenance of skeletal muscle homeostasis and function.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Minners J, Van Den Bos EJ, Yellon DM, Schwalb H, Opie LH, Sack MN. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: Support for a mitochondrial role in cardioprotection. Cardiovasc Res. 2000;47:68–73. doi: 10.1016/S0008-6363(00)00069-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RF-2016-02363566/Ministero della Salute (Ministry of Health, Italy)
- PRIN 2020R2BP2E_002/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- PRIN 2017YF9FBS/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- PRIN 2020RRJP5L/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- 22493/AFM-Téléthon (French Muscular Dystrophy Association)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

