Upregulated hepatic lipogenesis from dietary sugars in response to low palmitate feeding supplies brain palmitate
- PMID: 38233416
- PMCID: PMC10794264
- DOI: 10.1038/s41467-023-44388-4
Upregulated hepatic lipogenesis from dietary sugars in response to low palmitate feeding supplies brain palmitate
Abstract
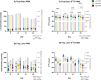
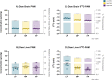
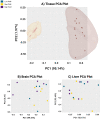
Palmitic acid (PAM) can be provided in the diet or synthesized via de novo lipogenesis (DNL), primarily, from glucose. Preclinical work on the origin of brain PAM during development is scarce and contrasts results in adults. In this work, we use naturally occurring carbon isotope ratios (13C/12C; δ13C) to uncover the origin of brain PAM at postnatal days 0, 10, 21 and 35, and RNA sequencing to identify the pathways involved in maintaining brain PAM, at day 35, in mice fed diets with low, medium, and high PAM from birth. Here we show that DNL from dietary sugars maintains the majority of brain PAM during development and is augmented in mice fed low PAM. Importantly, the upregulation of hepatic DNL genes, in response to low PAM at day 35, demonstrates the presence of a compensatory mechanism to maintain total brain PAM pools compared to the liver; suggesting the importance of brain PAM regulation.
© 2024. The Author(s).
Conflict of interest statement
R.P.B. is supported by grant funding through the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada and holds a Canada Research Chair in Brain Lipid Metabolism. R.P.B. has received industrial grants, including those matched by the Canadian government, and/or travel support related to work on brain fatty acid uptake from Arctic Nutrition, Bunge Ltd., Capsoil Technologies, DSM, Fonterra, Mead Johnson, Natures Crops International, and Nestec Inc. Moreover, R.P.B. and C.T.C. are on the executive committee of the International Society for the Study of Fatty Acids and Lipids and held a meeting on behalf of fatty acids and cell signaling, both of which rely on corporate sponsorship. R.P.B. has given expert testimony in relation to supplements and the brain. There was no role of funders in the conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript. The remaining authors declare no competing interests.
Figures



Similar articles
-
The majority of brain palmitic acid is maintained by lipogenesis from dietary sugars and is augmented in mice fed low palmitic acid levels from birth.J Neurochem. 2022 Apr;161(2):112-128. doi: 10.1111/jnc.15539. Epub 2021 Dec 11. J Neurochem. 2022. PMID: 34780089
-
Elevated de novo lipogenesis, slow liver triglyceride turnover, and clinical correlations in nonalcoholic steatohepatitis patients.J Lipid Res. 2022 Sep;63(9):100250. doi: 10.1016/j.jlr.2022.100250. Epub 2022 Jul 11. J Lipid Res. 2022. PMID: 35835205 Free PMC article.
-
Dietary sugar restriction reduces hepatic de novo lipogenesis in adolescent boys with fatty liver disease.J Clin Invest. 2021 Dec 15;131(24):e150996. doi: 10.1172/JCI150996. J Clin Invest. 2021. PMID: 34907907 Free PMC article. Clinical Trial.
-
Dietary carbohydrates and fatty liver disease: de novo lipogenesis.Curr Opin Clin Nutr Metab Care. 2018 Jul;21(4):277-282. doi: 10.1097/MCO.0000000000000469. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29697539 Review.
-
Conversion of Sugar to Fat: Is Hepatic de Novo Lipogenesis Leading to Metabolic Syndrome and Associated Chronic Diseases?J Am Osteopath Assoc. 2017 Aug 1;117(8):520-527. doi: 10.7556/jaoa.2017.102. J Am Osteopath Assoc. 2017. PMID: 28759094 Review.
Cited by
-
Fatty acid synthase global inducible knockout does not alter brain fatty acid concentrations but attenuates cholesterol synthesis in the adult mouse.Prostaglandins Leukot Essent Fatty Acids. 2025 Jul;205:102679. doi: 10.1016/j.plefa.2025.102679. Epub 2025 Mar 31. Prostaglandins Leukot Essent Fatty Acids. 2025. PMID: 40185011
References
-
- Lacombe, R. J. S. et al. Quantitative and carbon isotope ratio analysis of fatty acids isolated from human brain hemispheres. J. Neurochem. 10.1111/jnc.15702 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

