The amygdala NT3-TrkC pathway underlies inter-individual differences in fear extinction and related synaptic plasticity
- PMID: 38233468
- PMCID: PMC11189811
- DOI: 10.1038/s41380-024-02412-z
The amygdala NT3-TrkC pathway underlies inter-individual differences in fear extinction and related synaptic plasticity
Abstract
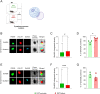
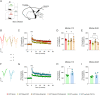
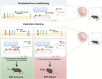
Fear-related pathologies are among the most prevalent psychiatric conditions, having inappropriate learned fear and resistance to extinction as cardinal features. Exposure therapy represents a promising therapeutic approach, the efficiency of which depends on inter-individual variation in fear extinction learning, which neurobiological basis is unknown. We characterized a model of extinction learning, whereby fear-conditioned mice were categorized as extinction (EXT)-success or EXT-failure, according to their inherent ability to extinguish fear. In the lateral amygdala, GluN2A-containing NMDAR are required for LTP and stabilization of fear memories, while GluN2B-containing NMDAR are required for LTD and fear extinction. EXT-success mice showed attenuated LTP, strong LTD and higher levels of synaptic GluN2B, while EXT-failure mice showed strong LTP, no LTD and higher levels of synaptic GluN2A. Neurotrophin 3 (NT3) infusion in the lateral amygdala was sufficient to rescue extinction deficits in EXT-failure mice. Mechanistically, activation of tropomyosin receptor kinase C (TrkC) with NT3 in EXT-failure slices attenuated lateral amygdala LTP, in a GluN2B-dependent manner. Conversely, blocking endogenous NT3-TrkC signaling with TrkC-Fc chimera in EXT-success slices strengthened lateral amygdala LTP. Our data support a key role for the NT3-TrkC system in inter-individual differences in fear extinction in rodents, through modulation of amygdalar NMDAR composition and synaptic plasticity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear.Neuropharmacology. 2012 Feb;62(2):797-806. doi: 10.1016/j.neuropharm.2011.09.001. Epub 2011 Sep 10. Neuropharmacology. 2012. PMID: 21925518
-
Forebrain GluN2A overexpression impairs fear extinction and NMDAR-dependent long-term depression in the lateral amygdala.Brain Res Bull. 2021 Sep;174:1-10. doi: 10.1016/j.brainresbull.2021.05.023. Epub 2021 May 28. Brain Res Bull. 2021. PMID: 34058285
-
Long-term potentiation at excitatory synaptic inputs to the intercalated cell masses of the amygdala.Int J Neuropsychopharmacol. 2014 Aug;17(8):1233-42. doi: 10.1017/S1461145714000133. Epub 2014 Feb 20. Int J Neuropsychopharmacol. 2014. PMID: 24556032
-
Out with the old and in with the new: Synaptic mechanisms of extinction in the amygdala.Brain Res. 2015 Sep 24;1621:231-8. doi: 10.1016/j.brainres.2014.10.010. Epub 2014 Oct 12. Brain Res. 2015. PMID: 25312830 Free PMC article. Review.
-
The role of the amygdala in the extinction of conditioned fear.Biol Psychiatry. 2006 Aug 15;60(4):322-8. doi: 10.1016/j.biopsych.2006.05.029. Biol Psychiatry. 2006. PMID: 16919522 Review.
Cited by
-
Synaptic accumulation of GluN2B-containing NMDA receptors mediates the effects of BDNF-TrkB signalling on synaptic plasticity and in hyperexcitability during status epilepticus.J Biomed Sci. 2025 Sep 1;32(1):82. doi: 10.1186/s12929-025-01164-4. J Biomed Sci. 2025. PMID: 40890771
-
Psilocybin-enhanced fear extinction linked to bidirectional modulation of cortical ensembles.Nat Neurosci. 2025 Jun;28(6):1311-1326. doi: 10.1038/s41593-025-01964-9. Epub 2025 May 26. Nat Neurosci. 2025. PMID: 40419686
References
-
- Michael T, Zetsche U, Margraf J. Epidemiology of anxiety disorders. Psychiatry. 2007;6:136–42. doi: 10.1016/j.mppsy.2007.01.007. - DOI
-
- American Psychiatric Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washigton DC, United States: American Psychiatric Pub; 2013, 1414pp.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

