INPP5A phosphatase is a synthetic lethal target in GNAQ and GNA11-mutant melanomas
- PMID: 38233483
- PMCID: PMC10965444
- DOI: 10.1038/s43018-023-00710-z
INPP5A phosphatase is a synthetic lethal target in GNAQ and GNA11-mutant melanomas
Abstract
Activating mutations in GNAQ/GNA11 occur in over 90% of uveal melanomas (UMs), the most lethal melanoma subtype; however, targeting these oncogenes has proven challenging and inhibiting their downstream effectors show limited clinical efficacy. Here, we performed genome-scale CRISPR screens along with computational analyses of cancer dependency and gene expression datasets to identify the inositol-metabolizing phosphatase INPP5A as a selective dependency in GNAQ/11-mutant UM cells in vitro and in vivo. Mutant cells intrinsically produce high levels of the second messenger inositol 1,4,5 trisphosphate (IP3) that accumulate upon suppression of INPP5A, resulting in hyperactivation of IP3-receptor signaling, increased cytosolic calcium and p53-dependent apoptosis. Finally, we show that GNAQ/11-mutant UM cells and patients' tumors exhibit elevated levels of IP4, a biomarker of enhanced IP3 production; these high levels are abolished by GNAQ/11 inhibition and correlate with sensitivity to INPP5A depletion. Our findings uncover INPP5A as a synthetic lethal vulnerability and a potential therapeutic target for GNAQ/11-mutant-driven cancers.
© 2024. The Author(s).
Conflict of interest statement
All authors except M.J.J., J.M.M.G. and M.P.L. are/were employees of Novartis Pharma during their time of contribution to this study. The authors declare no competing interests.
Figures



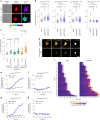


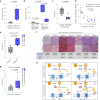
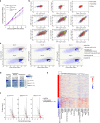

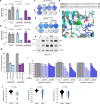
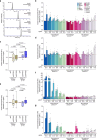
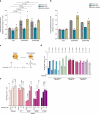
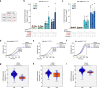
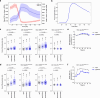


References
-
- Jager MJ, et al. Uveal melanoma. Nat. Rev. Dis. Primers. 2020;20:18–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

