Static magnetic stimulation induces structural plasticity at the axon initial segment of inhibitory cortical neurons
- PMID: 38233493
- PMCID: PMC10794225
- DOI: 10.1038/s41598-024-51845-7
Static magnetic stimulation induces structural plasticity at the axon initial segment of inhibitory cortical neurons
Abstract
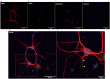
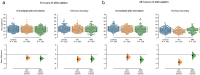
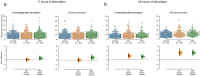
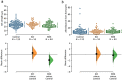
Static magnetic stimulation (SMS) is a form of non-invasive brain stimulation that alters neural activity and induces neural plasticity that outlasts the period of stimulation. This can modify corticospinal excitability or motor behaviours, suggesting that SMS may alter the intrinsic excitability of neurons. In mammalian neurons, the axon initial segment (AIS) is the site of action potential initiation and undergoes structural plasticity (changes in length and position from the soma) as a homeostatic mechanism to counteract chronic changes in neuronal activity. We investigated whether the chronic application of SMS (6 and 48 h, 0.5 T) induces structural AIS plasticity in postnatally derived primary cortical neurons. Following 6 h of SMS, we observed a shortening in mean AIS length compared to control, that persisted 24 h post stimulation. In contrast, 48 h of SMS induced an immediate distal shift that persisted 24 h post-stimulation. Pharmacological blockade of voltage gated L/T-type calcium channels during stimulation did not prevent SMS-induced AIS structural plasticity. Our findings provide the foundation to expand the use of chronic SMS as a non-invasive method to promote AIS plasticity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Theoretical relation between axon initial segment geometry and excitability.Elife. 2020 Mar 30;9:e53432. doi: 10.7554/eLife.53432. Elife. 2020. PMID: 32223890 Free PMC article.
-
Plasticity of the Axon Initial Segment: Fast and Slow Processes with Multiple Functional Roles.Neuroscientist. 2017 Aug;23(4):364-373. doi: 10.1177/1073858416648311. Epub 2016 May 3. Neuroscientist. 2017. PMID: 27143656 Review.
-
Motor learning changes the axon initial segment of the spinal motoneuron.J Physiol. 2024 May;602(9):2107-2126. doi: 10.1113/JP283875. Epub 2024 Apr 3. J Physiol. 2024. PMID: 38568869 Free PMC article.
-
Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability.Nature. 2010 Jun 24;465(7301):1070-4. doi: 10.1038/nature09160. Epub 2010 Jun 13. Nature. 2010. PMID: 20543823 Free PMC article.
-
The dynamic axon initial segment: From neuronal polarity to network homeostasis.Neuron. 2025 Mar 5;113(5):649-669. doi: 10.1016/j.neuron.2025.01.004. Epub 2025 Feb 12. Neuron. 2025. PMID: 39947181 Review.
Cited by
-
Single-domain magnetic particles with motion behavior under electromagnetic AC and DC fields are a fatal cargo in Metropolitan Mexico City pediatric and young adult early Alzheimer, Parkinson, frontotemporal lobar degeneration and amyotrophic lateral sclerosis and in ALS patients.Front Hum Neurosci. 2024 Aug 23;18:1411849. doi: 10.3389/fnhum.2024.1411849. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39246712 Free PMC article.
-
FMRP Controls Neuronal Architecture and Synaptic Content of NMDA Receptors in Cultured Hippocampal Neurons.J Mol Neurosci. 2025 Apr 2;75(2):44. doi: 10.1007/s12031-025-02325-8. J Mol Neurosci. 2025. PMID: 40172581 Free PMC article.
-
Effects of moderate static magnetic fields on voltage-gated potassium ion channels in sympathetic neuron-like PC12 cells.Physiol Rep. 2025 Mar;13(6):e70236. doi: 10.14814/phy2.70236. Physiol Rep. 2025. PMID: 40119575 Free PMC article.
-
Opportunities and obstacles in non-invasive brain stimulation.Front Hum Neurosci. 2024 Mar 18;18:1385427. doi: 10.3389/fnhum.2024.1385427. eCollection 2024. Front Hum Neurosci. 2024. PMID: 38562225 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

