Top-down control of flight by a non-canonical cortico-amygdala pathway
- PMID: 38233522
- PMCID: PMC10878556
- DOI: 10.1038/s41586-023-06912-w
Top-down control of flight by a non-canonical cortico-amygdala pathway
Abstract
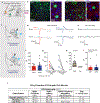
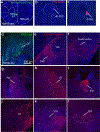
Survival requires the selection of appropriate behaviour in response to threats, and dysregulated defensive reactions are associated with psychiatric illnesses such as post-traumatic stress and panic disorder1. Threat-induced behaviours, including freezing and flight, are controlled by neuronal circuits in the central amygdala (CeA)2; however, the source of neuronal excitation of the CeA that contributes to high-intensity defensive responses is unknown. Here we used a combination of neuroanatomical mapping, in vivo calcium imaging, functional manipulations and electrophysiology to characterize a previously unknown projection from the dorsal peduncular (DP) prefrontal cortex to the CeA. DP-to-CeA neurons are glutamatergic and specifically target the medial CeA, the main amygdalar output nucleus mediating conditioned responses to threat. Using a behavioural paradigm that elicits both conditioned freezing and flight, we found that CeA-projecting DP neurons are activated by high-intensity threats in a context-dependent manner. Functional manipulations revealed that the DP-to-CeA pathway is necessary and sufficient for both avoidance behaviour and flight. Furthermore, we found that DP neurons synapse onto neurons within the medial CeA that project to midbrain flight centres. These results elucidate a non-canonical top-down pathway regulating defensive responses.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Figures













References
-
- Fadok JP et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–99 (2017). - PubMed
-
- Blanchard DC & Blanchard RJ Defensive behaviors, fear, and anxiety. in Handbook of anxiety and fear 63–79 (Elsevier Academic Press, 2008). doi:10.1016/S1569-7339(07)00005-7. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

