Dorsal peduncular cortex activity modulates affective behavior and fear extinction in mice
- PMID: 38233571
- PMCID: PMC11039686
- DOI: 10.1038/s41386-024-01795-5
Dorsal peduncular cortex activity modulates affective behavior and fear extinction in mice
Abstract
The medial prefrontal cortex (mPFC) is critical to cognitive and emotional function and underlies many neuropsychiatric disorders, including mood, fear and anxiety disorders. In rodents, disruption of mPFC activity affects anxiety- and depression-like behavior, with specialized contributions from its subdivisions. The rodent mPFC is divided into the dorsomedial prefrontal cortex (dmPFC), spanning the anterior cingulate cortex (ACC) and dorsal prelimbic cortex (PL), and the ventromedial prefrontal cortex (vmPFC), which includes the ventral PL, infralimbic cortex (IL), and in some studies the dorsal peduncular cortex (DP) and dorsal tenia tecta (DTT). The DP/DTT have recently been implicated in the regulation of stress-induced sympathetic responses via projections to the hypothalamus. While many studies implicate the PL and IL in anxiety-, depression-like and fear behavior, the contribution of the DP/DTT to affective and emotional behavior remains unknown. Here, we used chemogenetics and optogenetics to bidirectionally modulate DP/DTT activity and examine its effects on affective behaviors, fear and stress responses in C57BL/6J mice. Acute chemogenetic activation of DP/DTT significantly increased anxiety-like behavior in the open field and elevated plus maze tests, as well as passive coping in the tail suspension test. DP/DTT activation also led to an increase in serum corticosterone levels and facilitated auditory fear extinction learning and retrieval. Activation of DP/DTT projections to the dorsomedial hypothalamus (DMH) acutely decreased freezing at baseline and during extinction learning, but did not alter affective behavior. These findings point to the DP/DTT as a new regulator of affective behavior and fear extinction in mice.
© 2024. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures


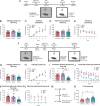
 indicates a significant difference between hM3D and hM4D groups (p < 0.05). F–L DP/DTT manipulation during fear extinction. F Experimental timeline. Mice previously injected with hM3D, hM4D or control mCherry constructs in the DP/DTT (no prior behavior) underwent fear acquisition (as previously; 6 tone-shock pairings). The following day, mice were injected with the DREADD agonist C21 one hour prior to undergoing extinction training. During extinction training, mice were placed in an alternate context (context B) and received 12 tone presentations. The following day, fear memory was evaluated during extinction retrieval, in the absence of C21. During extinction retrieval, mice were placed in context B and received 5 tone presentations. During auditory fear acquisition, mice from all groups displayed similar average tone freezing (G; one-way ANOVA F2,31 = 0.5886, p = 0.5612) but decreased freezing in tone 6 in the hM3D group (H; Two-way rmANOVA significant effect of tone F5,155 = 74.69, p < 0.0001 and interaction F10,155 = 1.984, p = 0.0385; but no main effect of treatment F2,31 = 0.8356, p = 0.4431; tone 6 mCherry vs hM3D p = 0.0023 and hM3D vs hM4D p = 0.0109). During extinction training, 1 h after C21 treatment, hM3D mice showed decreased average tone freezing (I; Two-way rmANOVA main effect of treatment and tone but no interaction F22,341 = 1.072, p = 0.3758) and tone-by-tone freezing (J) compared to hM4D and mCherry control mice. During extinction retrieval, hM3D mice froze less compared to hM4D and mCherry control mice in the average tone (K) and tone-by-tone (L; Two-way rmANOVA significant main effect of treatment and tone but no interaction F8,124 = 0.6037, p = 0.7733) analyses. Minimal sex differences were found in this dataset (see Supplementary Materials). M Experimental timeline for serum corticosterone assay. Mice previously injected with hM3D, hM4D or control mCherry constructs in the DP/DTT were injected with the DREADD agonist C21 and euthanized 90 minutes later. Blood was extracted at the time of perfusion for corticosterone measurements. N Serum corticosterone was significantly higher in hM3D mice compared to mCherry mice (but no change between mCherry and hM4D, p = 0.440, hM4D vs hM3D, p = 0.103).
indicates a significant difference between hM3D and hM4D groups (p < 0.05). F–L DP/DTT manipulation during fear extinction. F Experimental timeline. Mice previously injected with hM3D, hM4D or control mCherry constructs in the DP/DTT (no prior behavior) underwent fear acquisition (as previously; 6 tone-shock pairings). The following day, mice were injected with the DREADD agonist C21 one hour prior to undergoing extinction training. During extinction training, mice were placed in an alternate context (context B) and received 12 tone presentations. The following day, fear memory was evaluated during extinction retrieval, in the absence of C21. During extinction retrieval, mice were placed in context B and received 5 tone presentations. During auditory fear acquisition, mice from all groups displayed similar average tone freezing (G; one-way ANOVA F2,31 = 0.5886, p = 0.5612) but decreased freezing in tone 6 in the hM3D group (H; Two-way rmANOVA significant effect of tone F5,155 = 74.69, p < 0.0001 and interaction F10,155 = 1.984, p = 0.0385; but no main effect of treatment F2,31 = 0.8356, p = 0.4431; tone 6 mCherry vs hM3D p = 0.0023 and hM3D vs hM4D p = 0.0109). During extinction training, 1 h after C21 treatment, hM3D mice showed decreased average tone freezing (I; Two-way rmANOVA main effect of treatment and tone but no interaction F22,341 = 1.072, p = 0.3758) and tone-by-tone freezing (J) compared to hM4D and mCherry control mice. During extinction retrieval, hM3D mice froze less compared to hM4D and mCherry control mice in the average tone (K) and tone-by-tone (L; Two-way rmANOVA significant main effect of treatment and tone but no interaction F8,124 = 0.6037, p = 0.7733) analyses. Minimal sex differences were found in this dataset (see Supplementary Materials). M Experimental timeline for serum corticosterone assay. Mice previously injected with hM3D, hM4D or control mCherry constructs in the DP/DTT were injected with the DREADD agonist C21 and euthanized 90 minutes later. Blood was extracted at the time of perfusion for corticosterone measurements. N Serum corticosterone was significantly higher in hM3D mice compared to mCherry mice (but no change between mCherry and hM4D, p = 0.440, hM4D vs hM3D, p = 0.103).  indicates a significant group difference between mCherry and hM3D conditions;
indicates a significant group difference between mCherry and hM3D conditions;  indicates a significant group difference between hM3D and hM4D conditions;
indicates a significant group difference between hM3D and hM4D conditions;  indicates a significant difference between hM3D and all other groups. *p < 0.05. Male (square) and female (circle) individual datapoints are displayed for transparency. Some figure diagrams were created with the assistance of BioRender.com.
indicates a significant difference between hM3D and all other groups. *p < 0.05. Male (square) and female (circle) individual datapoints are displayed for transparency. Some figure diagrams were created with the assistance of BioRender.com.

References
Publication types
MeSH terms
Substances
Grants and funding
- CGSD3-534884-2019/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- RGPIN-2017-06344/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- PJT 399790/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- CDA00009/2018 and RGY0072/2019/Human Frontier Science Program (HFSP)
- NI19-1132R/Sick Kids Foundation
LinkOut - more resources
Full Text Sources

