Impact of tumor microenvironment on efficacy of anti-CD19 CAR T cell therapy or chemotherapy and transplant in large B cell lymphoma
- PMID: 38233586
- PMCID: PMC10878966
- DOI: 10.1038/s41591-023-02754-1
Impact of tumor microenvironment on efficacy of anti-CD19 CAR T cell therapy or chemotherapy and transplant in large B cell lymphoma
Abstract
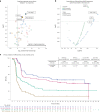
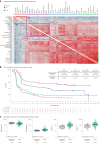
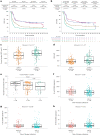
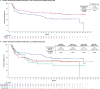
The phase 3 ZUMA-7 trial in second-line large B cell lymphoma demonstrated superiority of anti-CD19 CAR T cell therapy (axicabtagene ciloleucel (axi-cel)) over standard of care (SOC; salvage chemotherapy followed by hematopoietic transplantation) ( NCT03391466 ). Here, we present a prespecified exploratory analysis examining the association between pretreatment tumor characteristics and the efficacy of axi-cel versus SOC. B cell gene expression signature (GES) and CD19 expression associated significantly with improved event-free survival for axi-cel (P = 0.0002 for B cell GES; P = 0.0165 for CD19 expression) but not SOC (P = 0.9374 for B cell GES; P = 0.5526 for CD19 expression). Axi-cel showed superior event-free survival over SOC irrespective of B cell GES and CD19 expression (P = 8.56 × 10-9 for B cell GES high; P = 0.0019 for B cell GES low; P = 3.85 × 10-9 for CD19 gene high; P = 0.0017 for CD19 gene low). Low CD19 expression in malignant cells correlated with a tumor GES consisting of immune-suppressive stromal and myeloid genes, highlighting the inter-relation between malignant cell features and immune contexture substantially impacting axi-cel outcomes. Tumor burden, lactate dehydrogenase and cell-of-origin impacted SOC more than axi-cel outcomes. T cell activation and B cell GES, which are associated with improved axi-cel outcome, decreased with increasing lines of therapy. These data highlight differences in resistance mechanisms to axi-cel and SOC and support earlier intervention with axi-cel.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: F.L.L. reports a consulting or advisory role for Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, Cowen, ecoR1, Emerging Therapy Solutions Gerson Lehman Group, GammaDelta Therapeutics, Iovance, Janssen, Kite, a Gilead Company, Legend Biotech, Novartis, Umoja and Wugen; research funding from Allogene, Kite and Novartis; and patents, royalties and other intellectual property in the field of cellular immunotherapy. S.F. reports employment and stock or ownership with Kite, a Gilead Company, and patents, royalties and other intellectual property from Tusk Therapeutics. J.C. reports employment with, leadership role in and travel support from Kyverna and Kite, a Gilead Company; stock or other ownership in Gilead Sciences; and research funding, patents, royalties and other intellectual property from Kite. S.V. reports employment with and research funding from Kite, a Gilead Company; and stock or other ownership in Gilead Sciences. R.P. reports employment and stock or other ownership with Veracyte. P.D. reports a consulting or advisory role for, honoraria from and speakers’ bureau participation for Bristol-Myers Squibb, Gilead Sciences and Novartis. B.T.H reports honoraria from, a consulting or advisory role for, research funding from and travel support from Kite, a Gilead Company. C.L. reports stock in Kadmon; honoraria from CareDX, Jazz, Kadmon and Kite, a Gilead Company; a consulting or advisory role for Fresenius Kabi; and research funding from Incyte. P.L.Z. reports a consultant role for MSD, Eusa Pharma and Novartis; speakers’ bureau participation for Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, Servier, MSD, TG Therapeutics, Takeda, Roche, Eusa Pharma, Kyowa Kirin, Novartis, Incyte, and Beigene; and advisory board participation for Secura Bio, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, Servier, Sandoz, MSD, TG Therapeutics, Takeda, Roche, Eusa Pharma, Kyowa Kirin, Novartis, ADC Therapeutics, Incyte and Beigene. N.K. reports honoraria from and a consulting or advisory role for Kite, a Gilead Company; and research funding from Neovii, Novartis and Riemser. A.L.-G. reports a consulting or advisory role for Celgene, Incyte, Kite, a Gilead company, Novartis, Roche and Takeda; research funding from Celgene, Kite and Roche; and travel, accommodation and expenses from Kite. H.G. and W.Z. have no relevant financial relationships to disclose. G.T. and F.M.M. report employment with Kite, a Gilead Company. J.B., C.T., M.S. and H.M. report employment with Kite, a Gilead Company; and stock or other ownership in Gilead Sciences. M.M. and P.C. report employment with Kite, a Gilead Company, and patents, royalties and other intellectual property, and travel support from Kite; and stock or other ownership with Gilead Sciences. A.B. reports former employment with Kite, a Gilead Company; current employment and a leadership role with Capstan Therapeutics; stock or other ownership with Capstan Therapeutics and Gilead Sciences; a consulting or advisory role for Cero Therapeutics and Elicio; and expert testimony for Gilead Sciences. R.S. reports employment with and stock or other ownership in Kite, a Gilead Company; and patents, royalties and other intellectual property from Atara and Kite. D.B. reports employment with Kite, a Gilead Company. J.G. reports employment with Veracyte; a leadership role and consulting or advisory role in Northwest Biotherapeutics and Lunaphore; stock or other ownership in and travel support from Veracyte; research funding from Imcheck Therapeutics and Veracyte; and patents, royalties or other intellectual property from Inserm.
Figures










Similar articles
-
Tumor gene expression signatures associated with outcome in large B-cell lymphoma treated with CD19-directed CAR T-cell therapy (axicabtagene ciloleucel).Front Oncol. 2025 Feb 27;15:1519473. doi: 10.3389/fonc.2025.1519473. eCollection 2025. Front Oncol. 2025. PMID: 40083872 Free PMC article.
-
Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium.J Clin Oncol. 2020 Sep 20;38(27):3119-3128. doi: 10.1200/JCO.19.02104. Epub 2020 May 13. J Clin Oncol. 2020. PMID: 32401634 Free PMC article.
-
Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma.N Engl J Med. 2022 Feb 17;386(7):640-654. doi: 10.1056/NEJMoa2116133. Epub 2021 Dec 11. N Engl J Med. 2022. PMID: 34891224 Clinical Trial.
-
Evolving Role of CAR T Cell Therapy in First- and Second-Line Treatment of Large B Cell Lymphoma.Curr Oncol Rep. 2023 Nov;25(11):1387-1396. doi: 10.1007/s11912-023-01466-6. Epub 2023 Oct 20. Curr Oncol Rep. 2023. PMID: 37861914 Review.
-
Axicabtagene ciloleucel for the treatment of relapsed/refractory B-cell non-Hodgkin's lymphomas.Drugs Today (Barc). 2018 Mar;54(3):187-198. doi: 10.1358/dot.2018.54.3.2776625. Drugs Today (Barc). 2018. PMID: 29771253 Review.
Cited by
-
Biomarkers of outcome in patients undergoing CD19 CAR-T therapy for large B cell lymphoma.Hemasphere. 2024 Aug 22;8(8):e130. doi: 10.1002/hem3.130. eCollection 2024 Aug. Hemasphere. 2024. PMID: 39175824 Free PMC article. Review.
-
ARI0003: Co-transduced CD19/BCMA dual-targeting CAR-T cells for the treatment of non-Hodgkin lymphoma.Mol Ther. 2025 Jan 8;33(1):317-335. doi: 10.1016/j.ymthe.2024.11.028. Epub 2024 Nov 19. Mol Ther. 2025. PMID: 39563035
-
Outcome correlates of approved CD19-targeted CAR T cells for large B cell lymphoma.Nat Rev Clin Oncol. 2025 Apr;22(4):241-261. doi: 10.1038/s41571-025-00992-5. Epub 2025 Feb 18. Nat Rev Clin Oncol. 2025. PMID: 39966627 Review.
-
Baseline immune state and T-cell clonal kinetics are associated with durable response to CAR-T therapy in large B-cell lymphoma.Blood. 2024 Dec 12;144(24):2490-2502. doi: 10.1182/blood.2024024381. Blood. 2024. PMID: 39241199
-
MYC networks associate with decreased CD8 T-cell presence in diffuse large B-cell lymphoma and may be addressed by the synergistic combination of AZD4573 and Selinexor - a preliminary analysis.Ann Hematol. 2025 Apr;104(4):2403-2416. doi: 10.1007/s00277-025-06298-x. Epub 2025 Mar 11. Ann Hematol. 2025. PMID: 40064656 Free PMC article.
References
-
- Locke FL, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 2022;386:640–654. - PubMed
-
- Park JH, et al. The highest prognostic impact of LDH among International Prognostic Indices (IPIs): an explorative study of five IPI factors among patients with DLBCL in the era of rituximab. Ann. Hematol. 2014;93:1755–1764. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

