Carbon-nanotube field-effect transistors for resolving single-molecule aptamer-ligand binding kinetics
- PMID: 38233588
- PMCID: PMC11229667
- DOI: 10.1038/s41565-023-01591-0
Carbon-nanotube field-effect transistors for resolving single-molecule aptamer-ligand binding kinetics
Abstract
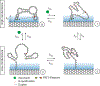
Small molecules such as neurotransmitters are critical for biochemical functions in living systems. While conventional ultraviolet-visible spectroscopy and mass spectrometry lack portability and are unsuitable for time-resolved measurements in situ, techniques such as amperometry and traditional field-effect detection require a large ensemble of molecules to reach detectable signal levels. Here we demonstrate the potential of carbon-nanotube-based single-molecule field-effect transistors (smFETs), which can detect the charge on a single molecule, as a new platform for recognizing and assaying small molecules. smFETs are formed by the covalent attachment of a probe molecule, in our case a DNA aptamer, to a carbon nanotube. Conformation changes on binding are manifest as discrete changes in the nanotube electrical conductance. By monitoring the kinetics of conformational changes in a binding aptamer, we show that smFETs can detect and quantify serotonin at the single-molecule level, providing unique insights into the dynamics of the aptamer-ligand system. In particular, we show the involvement of G-quadruplex formation and the disruption of the native hairpin structure in the conformational changes of the serotonin-aptamer complex. The smFET is a label-free approach to analysing molecular interactions at the single-molecule level with high temporal resolution, providing additional insights into complex biological processes.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
K.L.S. and E.F.Y. are involved in the commercialization of smFET devices for molecular diagnostic applications through Quicksilver Biosciences, Inc. The other authors declare no competing interests.
Figures













References
-
- Meneses A & Liy-Salmeron G Serotonin and emotion, learning and memory. Rev. Neurosci 23, 543–553 (2012). - PubMed
-
- Hendrickx S et al. A sensitive capillary LC-UV method for the simultaneous analysis of olanzapine, chlorpromazine and their FMO-mediated N-oxidation products in brain microdialysates. Talanta 162, 268–277 (2017). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

