Impaired humoral immunity following COVID-19 vaccination in HTLV-1 carriers
- PMID: 38233756
- PMCID: PMC10792913
- DOI: 10.1186/s12879-024-09001-z
Impaired humoral immunity following COVID-19 vaccination in HTLV-1 carriers
Abstract
Background: Whether human T-lymphotropic virus type 1 (HTLV-1) carriers can develop sufficient humoral immunity after coronavirus disease 2019 (COVID-19) vaccination is unknown.
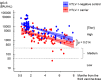
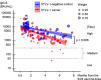
Methods: To investigate humoral immunity after COVID-19 vaccination in HTLV-1 carriers, a multicenter, prospective observational cohort study was conducted at five institutions in southwestern Japan, an endemic area for HTLV-1. HTLV-1 carriers and HTLV-1-negative controls were enrolled for this study from January to December 2022. During this period, the third dose of the COVID-19 vaccine was actively administered. HTLV-1 carriers were enrolled during outpatient visits, while HTLV-1-negative controls included health care workers and patients treated by participating institutions for diabetes, hypertension, or dyslipidemia. The main outcome was the effect of HTLV-1 infection on the plasma anti-COVID-19 spike IgG (IgG-S) titers after the third dose, assessed by multivariate linear regression with other clinical factors.
Results: We analyzed 181 cases (90 HTLV-1 carriers, 91 HTLV-1-negative controls) after receiving the third dose. HTLV-1 carriers were older (median age 67.0 vs. 45.0 years, p < 0.001) and more frequently had diabetes, hypertension, or dyslipidemia than did HTLV-1-negative controls (60.0% vs. 27.5%, p < 0.001). After the third dose, the IgG-S titers decreased over time in both carriers and controls. Multivariate linear regression in the entire cohort showed that time since the third dose, age, and HTLV-1 infection negatively influenced IgG-S titers. After adjusting for confounders such as age, or presence of diabetes, hypertension, or dyslipidemia between carriers and controls using the overlap weighting propensity score method, and performing weighted regression analysis in the entire cohort, both time since the third dose and HTLV-1 infection negatively influenced IgG-S titers.
Conclusions: The humoral immunity after the third vaccination dose is impaired in HTLV-1 carriers; thus, customized vaccination schedules may be necessary for them.
Keywords: COVID-19; HTLV-1; Humoral immunity; SARS-COV-2 spike protein; Vaccination.
© 2024. The Author(s).
Conflict of interest statement
K. Shimoda received consulting fees from Novartis Pharma, Takeda Pharmaceutical, and Bristol-Myers, all outside the submitted work, and received research grants from Perseus Proteomics, Pharma Essentia Japan KK, AbbVie GK, Astellas Pharma, MSD, Chugai Pharmaceutical, Kyowa Kirin, Pfizer, Novartis Pharma, Otsuka Pharmaceutical, and Asahi Kasei Medical, all outside the submitted work. A. Utsunomiya has received honoraria from Kyowa Kirin, Daiichi Sankyo, Bristol-Myers Squibb, and Meiji Seika Pharma and consulting fees from JIMRO and Otsuka Medical Devices, all outside the submitted work. M. Hidaka received honoraria from Chugai Pharm. And Huya, Japan. The authors declare no conflicts of interest.
Figures
Similar articles
-
Immune response to COVID-19 vaccines among people living with human T-cell lymphotropic virus type 1 infection: a retrospective cohort study from Iran.J Neurovirol. 2025 Feb;31(1):35-40. doi: 10.1007/s13365-023-01176-6. Epub 2023 Oct 23. J Neurovirol. 2025. PMID: 37870718
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
-
[Humoral immunity against SARS-CoV-2 in workers of social health care centers of Castilla y León after vaccination with the BNT162b2 mRNA vaccine from Pfizer/Biontech.].Rev Esp Salud Publica. 2021 Oct 25;95:e202110141. Rev Esp Salud Publica. 2021. PMID: 34690347 Spanish.
-
Blunted humoral immune response to the fourth dose of BNT162b2 COVID-19 vaccine in patients undergoing hemodialysis.Clin Exp Nephrol. 2023 Jul;27(7):639-647. doi: 10.1007/s10157-023-02342-0. Epub 2023 Mar 28. Clin Exp Nephrol. 2023. PMID: 36977892 Free PMC article.
-
One-month humoral response following two or three doses of messenger RNA coronavirus disease 2019 vaccines as primary vaccination in specific populations in France: first results from the Agence Nationale Recherche contre le Sida (ANRS)0001S COV-POPART cohort.Clin Microbiol Infect. 2023 Mar;29(3):388.e1-388.e8. doi: 10.1016/j.cmi.2022.10.009. Epub 2022 Oct 14. Clin Microbiol Infect. 2023. PMID: 36252789 Free PMC article.
Cited by
-
Seroprevalence of Strongyloides stercoralis, human T-lymphotropic virus, and Chagas disease in the Peruvian Amazon: a cross-sectional study.Rev Inst Med Trop Sao Paulo. 2024 Dec 16;66:e73. doi: 10.1590/S1678-9946202466073. eCollection 2024. Rev Inst Med Trop Sao Paulo. 2024. PMID: 39699511 Free PMC article.
References
-
- Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, Curigliano G, de Azambuja E. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. - DOI - PMC - PubMed
-
- Lee LYW, Cazier JB, Starkey T, Briggs SEW, Arnold R, Bisht V, Booth S, Campton NA, Cheng VWT, Collins G, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309–16. doi: 10.1016/S1470-2045(20)30442-3. - DOI - PMC - PubMed
-
- Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, Martin-Moro F, Razanamahery J, Riches JC, Zwicker J, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–92. doi: 10.1182/blood.2020008824. - DOI - PMC - PubMed
-
- Okamoto A, Fujigaki H, Iriyama C, Goto N, Yamamoto H, Mihara K, Inaguma Y, Miura Y, Furukawa K, Yamamoto Y, et al. CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma. Blood Adv. 2022;6(11):3230–3. doi: 10.1182/bloodadvances.2021006302. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

