ZDHHC5-mediated S-palmitoylation of FAK promotes its membrane localization and epithelial-mesenchymal transition in glioma
- PMID: 38233791
- PMCID: PMC10795333
- DOI: 10.1186/s12964-023-01366-z
ZDHHC5-mediated S-palmitoylation of FAK promotes its membrane localization and epithelial-mesenchymal transition in glioma
Abstract
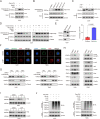
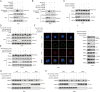
Background: Abnormal activation of FAK is associated with tumor development and metastasis. Through interactions with other intracellular signalling molecules, FAK influences cytoskeletal remodelling, modulation of adhesion signalling, and activation of transcription factors, promoting migration and invasion of tumor cells. However, the exact mechanism that regulates these processes remains unresolved. Herein, our findings indicate that the S-palmitoylation of FAK is crucial for both its membrane localization and activation.
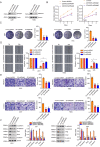
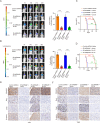
Methods: The palmitoylation of FAK in U251 and T98G cells was assessed by an acyl-PEG exchange (APE) assay and a metabolic incorporation assay. Cellular palmitoylation was inhibited using 2-bromopalmitate, and the palmitoylation status and cellular localization of FAK were determined. A metabolic incorporation assay was used to identify the potential palmitoyl acyltransferase and the palmitoylation site of FAK. Cell Counting Kit-8 (CCK8) assays, colony formation assays, and Transwell assays were conducted to assess the impact of ZDHHC5 in GBM. Additionally, intracranial GBM xenografts were utilized to investigate the effects of genetically silencing ZDHHC5 on tumor growth.
Results: Inhibiting FAK palmitoylation leads to its redistribution from the membrane to the cytoplasm and a decrease in its phosphorylation. Moreover, ZDHHC5, a protein-acyl-transferase (PAT), catalyzes this key modification of FAK at C456. Knockdown of ZDHHC5 abrogates the S-palmitoylation and membrane distribution of FAK and impairs cell proliferation, invasion, and epithelial-mesenchymal transition (EMT). Taken together, our research reveals the crucial role of ZDHHC5 as a PAT responsible for FAK S-palmitoylation, membrane localization, and activation.
Conclusions: These results imply that targeting the ZDHHC5/FAK axis has the potential to be a promising strategy for therapeutic interventions for glioblastoma (GBM). Video Abstract.
Keywords: FAK ZDHHC5 S; Mesenchymal transition; Palmitoylation glioma epithelial.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no potential competing interests.
Figures


Similar articles
-
Research progress on S-palmitoylation modification mediated by the ZDHHC family in glioblastoma.Front Cell Dev Biol. 2024 Nov 5;12:1413708. doi: 10.3389/fcell.2024.1413708. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39563863 Free PMC article. Review.
-
The interactions of ZDHHC5/GOLGA7 with SARS-CoV-2 spike (S) protein and their effects on S protein's subcellular localization, palmitoylation and pseudovirus entry.Virol J. 2021 Dec 27;18(1):257. doi: 10.1186/s12985-021-01722-w. Virol J. 2021. PMID: 34961524 Free PMC article.
-
Propofol enhances stem-like properties of glioma via GABAAR-dependent Src modulation of ZDHHC5-EZH2 palmitoylation mechanism.Stem Cell Res Ther. 2022 Aug 4;13(1):398. doi: 10.1186/s13287-022-03087-5. Stem Cell Res Ther. 2022. PMID: 35927718 Free PMC article.
-
Flotillin-1 palmitoylation is essential for its stability and subsequent tumor promoting capabilities.Oncogene. 2024 Mar;43(14):1063-1074. doi: 10.1038/s41388-024-02946-0. Epub 2024 Feb 19. Oncogene. 2024. PMID: 38374406
-
Regulation and function of the palmitoyl-acyltransferase ZDHHC5.FEBS J. 2021 Dec;288(23):6623-6634. doi: 10.1111/febs.15709. Epub 2021 Jan 20. FEBS J. 2021. PMID: 33415776 Review.
Cited by
-
QSOX1 Modulates Glioblastoma Cell Proliferation and Migration In Vitro and Invasion In Vivo.Cancers (Basel). 2024 Oct 26;16(21):3620. doi: 10.3390/cancers16213620. Cancers (Basel). 2024. PMID: 39518060 Free PMC article.
-
Research progress on S-palmitoylation modification mediated by the ZDHHC family in glioblastoma.Front Cell Dev Biol. 2024 Nov 5;12:1413708. doi: 10.3389/fcell.2024.1413708. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39563863 Free PMC article. Review.
-
zDHHC3-mediated S-palmitoylation of SLC9A2 regulates apoptosis in kidney clear cell carcinoma.J Cancer Res Clin Oncol. 2024 Apr 15;150(4):194. doi: 10.1007/s00432-024-05737-y. J Cancer Res Clin Oncol. 2024. PMID: 38619631 Free PMC article.
-
Palmitoylation in cardiovascular diseases: Molecular mechanism and therapeutic potential.Int J Cardiol Heart Vasc. 2025 Apr 4;58:101675. doi: 10.1016/j.ijcha.2025.101675. eCollection 2025 Jun. Int J Cardiol Heart Vasc. 2025. PMID: 40242212 Free PMC article. Review.
-
Research trends of protein palmitoylation in cancer from 2004 to 2024: a bibliometric and visualization analysis.Front Oncol. 2025 Jun 23;15:1571870. doi: 10.3389/fonc.2025.1571870. eCollection 2025. Front Oncol. 2025. PMID: 40626019 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

