Tacrolimus versus mycophenolate for AutoImmune hepatitis patients with incompLete response On first-line therapy (TAILOR study): a study protocol for a phase III, open-label, multicentre, randomised controlled trial
- PMID: 38233878
- PMCID: PMC10792789
- DOI: 10.1186/s13063-023-07832-w
Tacrolimus versus mycophenolate for AutoImmune hepatitis patients with incompLete response On first-line therapy (TAILOR study): a study protocol for a phase III, open-label, multicentre, randomised controlled trial
Abstract
Background: Autoimmune hepatitis (AIH) is a rare, chronic inflammatory disease of the liver. The treatment goal is reaching complete biochemical response (CR), defined as the normalisation of aspartate and alanine aminotransferases and immunoglobulin gamma. Ongoing AIH activity can lead to fibrosis and (decompensated) cirrhosis. Incomplete biochemical response is the most important risk factor for liver transplantation or liver-related mortality. First-line treatment consists of a combination of azathioprine and prednisolone. If CR is not reached, tacrolimus (TAC) or mycophenolate mofetil (MMF) can be used as second-line therapy. Both products are registered for the prevention of graft rejection in solid organ transplant recipients. The aim of this study is to compare the effectiveness and safety of TAC and MMF as second-line treatment for AIH.
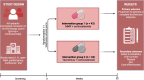
Methods: The TAILOR study is a phase IIIB, multicentre, open-label, parallel-group, randomised (1:1) controlled trial performed in large teaching and university hospitals in the Netherlands. We will enrol 86 patients with AIH who have not reached CR after at least 6 months of treatment with first-line therapy. Patients are randomised to TAC (0.07 mg/kg/day initially and adjusted by trough levels) or MMF (max 2000 mg/day), stratified by the presence of cirrhosis at inclusion. The primary endpoint is the difference in the proportion of patients reaching CR after 12 months. Secondary endpoints include the difference in the proportion of patients reaching CR after 6 months, adverse effects, difference in fibrogenesis, quality of life and cost-effectiveness.
Discussion: This is the first randomised controlled trial comparing two second-line therapies for AIH. Currently, second-line treatment is based on retrospective cohort studies. The rarity of AIH is the main issue in clinical research for alternative treatment options. The results of this trial can be implemented in existing international clinical guidelines.
Trial registration: ClinicalTrials.gov NCT05221411 . Retrospectively registered on 3 February 2022; EudraCT number 2021-003420-33. Prospectively registered on 16 June 2021.
Keywords: AIH; Autoimmune hepatitis; Autoimmune liver disease; Complete biochemical response; Mycophenolate mofetil; Randomised controlled trials; Second-line treatment; Tacrolimus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests. Although the LUMC pharmacy procures Envarsus at a discounted rate, this arrangement has no bearing on the study and is unrelated to the content of this manuscript.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical