Radiological diagnosis of hepatocellular carcinoma does not preclude biopsy before treatment
- PMID: 38234407
- PMCID: PMC10792651
- DOI: 10.1016/j.jhepr.2023.100957
Radiological diagnosis of hepatocellular carcinoma does not preclude biopsy before treatment
Abstract
Background & aims: The diagnosis of hepatocellular carcinoma (HCC) in patients with cirrhosis relies on non-invasive criteria based on international guidelines. The advent of systemic therapies warrants reconsideration of the role of biopsy specimens in the diagnosis of HCC. Accordingly, we investigated the diagnostic performance of the LI-RADS 2018 and the AASLD 2011 criteria.
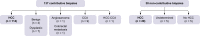
Methods: Consecutive patients with cirrhosis who underwent a biopsy for suspected HCC between 2015 and 2020 were included. The available imaging studies (computed tomography and/or magnetic resonance imaging) were blindly reviewed by two independent radiologists. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were assessed for LI-RADS, AASLD, and biopsies.
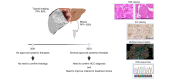
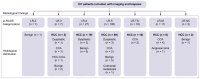
Results: In total, 167 patients underwent both available biopsy and imaging. Of the 137 relevant biopsies, 114 patients had HCC (83.2%), 12 (9%) had non-HCC malignant lesions, and 11 (8%) had benign nodules. The PPV and NPV of the biopsies were 100% and 62%, respectively; 30 biopsies were non-contributive. The PPV and NPV of the LI-RADS categories were 89% and 32.8% for LR-5 and 85.5% and 54.5% for LR-4 + 5 + TIV, respectively. The PPV and NPV of the 2011 AASLD criteria were 93.2% and 35.6%, respectively. The interobserver kappa (k = 0.380) for the LR-5 categories was reasonable. Of 100 LR-5 nodules, 11 were misclassified, in particular one case was a colorectal metastasis, and two cases were cholangiocarcinomas, of which nine were identified through biopsy, whereas six were correctly classified according to LI-RADS (LR-M or LR-TIV). Fifty percent of macrotrabecular HCC and 48.4% of poorly differentiated HCC (Edmonson 3 and 4) were not classified as LR-5.
Conclusions: LI-RADS 2018 did not outperform the AASLD 2011 score as a non-invasive diagnosis of HCC. Tumor biopsy allowed restoration of an accurate diagnosis in 11% of LR-5 cases. A combined radiological and histological diagnosis should be considered mandatory for good treatment assessment.
Impact and implications: Although biopsy is not required for hepatocellular carcinoma diagnosis when the LI-RADS criteria are met according to current guidelines, our study underscores the limits of radiology and the need for biopsy when hepatocellular carcinoma is suspected. Histological findings could change therapeutics of liver tumors even if only for a small proportion of patients. Histological proof of the type of cancer is a standard in oncology.
Keywords: American Association for the study of Liver Diseases 2011; Biopsy; Diagnostic performance; Hepatocellular carcinoma; LI-RADS V2018.
© 2023 The Author(s).
Conflict of interest statement
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Ioannou G.N., Bryson C.L., Weiss N.S., et al. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57:249–257. - PubMed
-
- European Association for Study of Liver, European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641. - PubMed
-
- Bruix J., Sherman M., Llovet J.M., et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol. 2001;35:421–430. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

