Acridones as promising drug candidates against Oropouche virus
- PMID: 38234431
- PMCID: PMC10792649
- DOI: 10.1016/j.crmicr.2023.100217
Acridones as promising drug candidates against Oropouche virus
Abstract
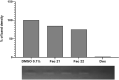
Oropouche virus (OROV) is an emerging vector-borne arbovirus found in South America that causes Oropouche fever, a febrile infection similar to dengue fever. It has a high epidemic potential, causing illness in over 500,000 cases diagnosed since the virus was first discovered in 1955. Currently, the prevention of human viral infection depends on vaccination, but availability for many viruses is limited, and they are classified as neglected viruses. At present, there are no vaccines or antiviral treatments available. An alternative approach to limiting the spread of the virus is to selectively disrupt viral replication mechanisms. Here, we demonstrate the inhibitory effect of acridones, which efficiently inhibited viral replication by 99.9 % in vitro. To evaluate possible mechanisms of action, we conducted tests with dsRNA, an intermediate in virus replication, as well as MD simulations, docking, and binding free energy analysis. The results showed a strong interaction between FAC21 and the OROV endonuclease, which possibly limits the interaction of viral RNA with other proteins. Therefore, our results suggest a dual mechanism of antiviral action, possibly caused by ds-RNA intercalation. In summary, our findings demonstrate that a new generation of antiviral drugs could be developed based on the selective optimization of molecules.
Keywords: Acridone; Antiviral; Drug discovery; Endonuclease; Oropouche.
© 2023 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mauricio Lacerda Nogueira reports financial support was provided by The Coordinating Research on Emerging Arboviral Threats Encompassing the Neotropics.
Figures
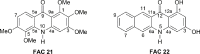





References
-
- Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
-
- Almeida G.M., Souza J.P., Mendes N.D., Pontelli M.C., Pinheiro N.R., Nogueira G.O., Cardoso R.S., Paiva I.M., Ferrari G.D., Veras F.P., Cunha F.Q., Horta-Junior J.A.C., Alberici L.C., Cunha T.M., Podolsky-Gondim G.G., Neder L., Arruda E., Sebollela A. Neural infection by oropouche virus in adult human brain slices induces an inflammatory and toxic response. Front. Neurosci. 2021:15. - PMC - PubMed
-
- Azevedo R.S., da S., Nunes M.R.T., Chiang J.O., Bensabath G., Vasconcelos H.B., Pinto A.Y., das N., Martins L.C., Monteiro H.A., de O., Rodrigues S.G., Vasconcelos P.F., da C. Reemergence of Oropouche fever, northern Brazil. Emerg. Infect. Dis. 2007;13:912–915. doi: 10.3201/eid1306.061114. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

